A review of the clinical experience with CMN-001, a tumor RNA loaded dendritic cell immunotherapy for the treatment of metastatic renal cell carcinoma
- PMID: 37387210
- PMCID: PMC10332239
- DOI: 10.1080/21645515.2023.2220629
A review of the clinical experience with CMN-001, a tumor RNA loaded dendritic cell immunotherapy for the treatment of metastatic renal cell carcinoma
Abstract
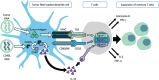
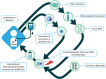
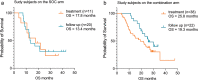
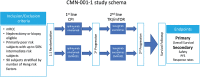
Engineering dendritic cells (DCs) to treat cancer is a long sought-after goal for cell-based immunotherapies. In this review, we focus on the experience with CMN-001, formally AGS-003, a DC-based immunotherapy, employing autologous DC electroporated with autologous tumor RNA to treat subjects with metastatic renal cell carcinoma (mRCC). We will review the early clinical development of CMN-001 up to and including deployment in a multicenter phase 3 study and provide a rationale to continue the development of CMN-001 in an ongoing randomized phase 2 study. The synergy between CMN-001 and everolimus observed in the phase 3 study provides an opportunity to design a phase 2b study building on the mechanism of action of CMN-001 and underlying immune and clinical outcomes revealed in the earlier studies. The design of the phase 2b study combines CMN-001 with first-line checkpoint inhibition therapy and second line lenvatinib/everolimus in poor-risk mRCC subjects.
Keywords: Dendritic cells; cancer vaccines; immunotherapy; mRCC; memory T cells.
Conflict of interest statement
Mark DeBenedette, Alicia Gamble, Marcus Norris, Joe Horvatinovich, and Charles A. Nicolette are all full-time employees of CoImmune Inc. and have stock options in the company.
Figures



References
-
- Chick RC, Faries MB, Hale DF, Kemp Bohan PM, Hickerson AT, Vreeland TJ, Myers JW, Cindass JL, Brown TA, Hyngstrom J, et al. Multi-institutional, prospective, randomized, double-blind, placebo-controlled phase IIb trial of the tumor lysate, particle-loaded, dendritic cell (TLPLDC) vaccine to prevent recurrence in high-risk melanoma patients: a subgroup analysis. Cancer Med. 2021;10:4302–14. doi: 10.1002/cam4.3969. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
