DnaJC7 specifically regulates tau seeding
- PMID: 37387473
- PMCID: PMC10473839
- DOI: 10.7554/eLife.86936
DnaJC7 specifically regulates tau seeding
Abstract
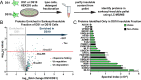
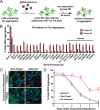
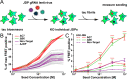
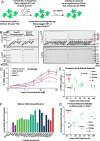
Neurodegenerative tauopathies are caused by accumulation of toxic tau protein assemblies. This appears to involve template-based seeding events, whereby tau monomer changes conformation and is recruited to a growing aggregate. Several large families of chaperone proteins, including Hsp70s and J domain proteins (JDPs), cooperate to regulate the folding of intracellular proteins such as tau, but the factors that coordinate this activity are not well known. The JDP DnaJC7 binds tau and reduces its intracellular aggregation. However, it is unknown whether this is specific to DnaJC7 or if other JDPs might be similarly involved. We used proteomics within a cell model to determine that DnaJC7 co-purified with insoluble tau and colocalized with intracellular aggregates. We individually knocked out every possible JDP and tested the effect on intracellular aggregation and seeding. DnaJC7 knockout decreased aggregate clearance and increased intracellular tau seeding. This depended on the ability of the J domain (JD) of DnaJC7 to stimulate Hsp70 ATPase activity, as JD mutations that block this interaction abrogated the protective activity. Disease-associated mutations in the JD and substrate binding site of DnaJC7 also abolished its protective activity. DnaJC7 thus specifically regulates tau aggregation in cooperation with Hsp70.
Keywords: DnaJC7; J domain protein; amyloid; biochemistry; chaperones; chemical biology; human; neuroscience; seeding; tau.
© 2023, Perez et al.
Conflict of interest statement
VP, DS, AM, BS, VM, CW, LJ, MD No competing interests declared
Figures





Update of
-
DnaJC7 specifically regulates tau seeding.bioRxiv [Preprint]. 2023 Mar 16:2023.03.16.532880. doi: 10.1101/2023.03.16.532880. bioRxiv. 2023. Update in: Elife. 2023 Jun 30;12:e86936. doi: 10.7554/eLife.86936. PMID: 36993367 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

