Toxoplasma gondii inhibits the expression of autophagy-related genes through AKT-dependent inactivation of the transcription factor FOXO3a
- PMID: 37387601
- PMCID: PMC10470550
- DOI: 10.1128/mbio.00795-23
Toxoplasma gondii inhibits the expression of autophagy-related genes through AKT-dependent inactivation of the transcription factor FOXO3a
Abstract
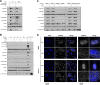
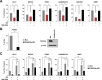
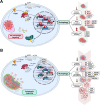
The intracellular parasite Toxoplasma gondii induces host AKT activation to prevent autophagy-mediated clearance; however, the molecular underpinnings are not fully understood. Autophagy can be negatively regulated through AKT-sensitive phosphorylation and nuclear export of the transcription factor Forkhead box O3a (FOXO3a). Using a combination of pharmacological and genetic approaches, herein we investigated whether T. gondii hinders host autophagy through AKT-dependent inactivation of FOXO3a. We found that infection by type I and II strains of T. gondii promotes gradual and sustained AKT-dependent phosphorylation of FOXO3a at residues S253 and T32 in human foreskin fibroblasts (HFF) and murine 3T3 fibroblasts. Mechanistically, AKT-sensitive phosphorylation of FOXO3a by T. gondii required live infection and the activity of PI3K but was independent of the plasma membrane receptor EGFR and the kinase PKCα. Phosphorylation of FOXO3a at AKT-sensitive residues was paralleled by its nuclear exclusion in T. gondii-infected HFF. Importantly, the parasite was unable to drive cytoplasmic localization of FOXO3a upon pharmacological blockade of AKT or overexpression of an AKT-insensitive mutant form of FOXO3a. Transcription of a subset of bona fide autophagy-related targets of FOXO3a was reduced during T. gondii infection in an AKT-dependent fashion. However, parasite-directed repression of autophagy-related genes was AKT-resistant in cells deficient in FOXO3a. Consistent with this, T. gondii failed to inhibit the recruitment of acidic organelles and LC3, an autophagy marker, to the parasitophorous vacuole upon chemically or genetically induced nuclear retention of FOXO3a. In all, we provide evidence that T. gondii suppresses FOXO3a-regulated transcriptional programs to prevent autophagy-mediated killing. IMPORTANCE The parasite Toxoplasma gondii is the etiological agent of toxoplasmosis, an opportunistic infection commonly transmitted by ingestion of contaminated food or water. To date, no effective vaccines in humans have been developed and no promising drugs are available to treat chronic infection or prevent congenital infection. T. gondii targets numerous host cell processes to establish a favorable replicative niche. Of note, T. gondii activates the host AKT signaling pathway to prevent autophagy-mediated killing. Herein, we report that T. gondii inhibits FOXO3a, a transcription factor that regulates the expression of autophagy-related genes, through AKT-dependent phosphorylation. The parasite's ability to block the recruitment of the autophagy machinery to the parasitophorous vacuole is impeded upon pharmacological inhibition of AKT or overexpression of an AKT-insensitive form of FOXO3a. Thus, our study provides greater granularity in the role of FOXO3a during infection and reinforces the potential of targeting autophagy as a therapeutic strategy against T. gondii.
Keywords: AKT; FOXO3a; Toxoplasma gondii; autophagy; host response; host-pathogen interactions; transcriptional regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

