Cyclic Allene Approach to the Manzamine Alkaloid Keramaphidin B
- PMID: 37387644
- PMCID: PMC10460088
- DOI: 10.1021/acs.orglett.3c01489
Cyclic Allene Approach to the Manzamine Alkaloid Keramaphidin B
Abstract
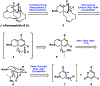
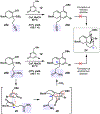
We report an approach to the core of the manzamine alkaloid keramaphidin B that relies on the strain-promoted cycloaddition of an azacyclic allene with a pyrone trapping partner. The cycloaddition is tolerant of nitrile and primary amide functional groups and can be complemented with a subsequent retro-Diels-Alder step. These efforts demonstrate that strained cyclic allenes can be used to build significant structural complexity and should encourage further studies of these fleeting intermediates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
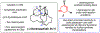




References
-
- Magnier E; Langlois Y Manzamine Alkaloids, Syntheses and Synthetic Approaches. Tetrahedron 1998, 54, 6201–6258.
-
- Radwan M; Hanora A; Khalifa S; Abou-El-Ela SH Manzamines. Cell Cycle 2012, 11, 1765–1772. - PubMed
-
- Ashok P; Ganguly S; Murugesan S Manzamine Alkaloids: Isolation, Cytotoxicity, Antimalarial Activity and SAR Studies. Drug Discovery Today 2014, 19, 1781–1791. - PubMed
-
- Kobayashi J. i.; Tsuda M; Kawasaki N; Matsumoto K; Adachi T Keramaphidin B, A Novel Pentacyclic Alkaloid from a Marine Sponge Amphimedon sp.: A Plausible Biogenetic Precursor of Manzamine Alkaloids. Tetrahedron Lett. 1994, 35, 4383–4386.
-
- Tsuda M; Inaba K; Kawasaki N; Honma K; Kobayashi J Chiral Resolution of (±)–Keramaphidin B and Isolation of Manzamine L, a New β-Carboline Alkaloid from a Sponge Amphimedon sp. Tetrahedron 1996, 52, 2319–2324.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

