A group of three miRNAs can act as candidate circulating biomarkers in liquid biopsies from melanoma patients
- PMID: 37387784
- PMCID: PMC10301821
- DOI: 10.3389/fmed.2023.1180799
A group of three miRNAs can act as candidate circulating biomarkers in liquid biopsies from melanoma patients
Abstract
Background: Staging of melanoma and follow up after melanoma diagnosis aims at predicting risk and detecting progression or recurrence at early stage, respectively in order to timely start and/or change treatment. Tumor thickness according to Breslow, status of the sentinel node and value of the lactate dehydrogenase (LDH) are well-established prognostic markers for metastatic risk, but reliable biomarkers identifying early recurrence or candidates who may benefit best from medical treatment are still warranted. Liquid biopsy has emerged to be a suitable method for identifying biomarkers for early cancer diagnosis, prognosis, therapeutic response prediction, and patient follow-up. Liquid biopsy is a blood-based non-invasive procedure that allows analyzing circulating analytes, including extracellular vesicles.
Methods: In this study we have explored the use of 7 miRNAs, namely hsa-miR-149-3p, hsa-miR-150-5p, hsa-miR-21-5p, hsa-miR-200c-3p, hsa-miR-134-5p, hsa-miR-144-3p and hsa-miR-221-3p in plasma exosomes to discriminate melanoma patients from controls without melanoma in a cohort of 92 individuals.
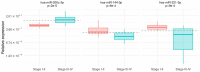
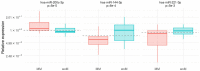
Results and discussion: Our results showed that three out seven miRNAs, namely hsa-miR-200c-3p, hsa-miR-144-3p and hsa-miR-221-3p were differentially expressed in plasma-derived exosomes from melanoma patients and controls. Furthermore, the expression of the three miRNAs may be a promising ancillary tool as a melanoma biomarker, even for discriminating between nevi and melanoma.
Keywords: exosomes; liquid biopsy; melanoma; miRNA; plasma.
Copyright © 2023 De Martino, Gandin, Azzalini, Massone, Pizzichetta, Giulioni, Javor, Pinzani, Conforti, Zalaudek and Bonin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

