A panel of seven protein tumour markers for effective and affordable multi-cancer early detection by artificial intelligence: a large-scale and multicentre case-control study
- PMID: 37387788
- PMCID: PMC10300313
- DOI: 10.1016/j.eclinm.2023.102041
A panel of seven protein tumour markers for effective and affordable multi-cancer early detection by artificial intelligence: a large-scale and multicentre case-control study
Abstract
Background: Early detection of cancer aims to reduce cancer deaths. Unfortunately, many established cancer screening technologies are not suitable for use in low- and middle-income countries (LMICs) due to cost, complexity, and dependency on extensive medical infrastructure. We aimed to assess the performance and robustness of a protein assay (OncoSeek) for multi-cancer early detection (MCED) that is likely to be more practical in LMICs.
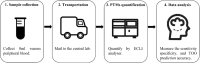
Methods: This observational study comprises a retrospective analysis on the data generated from the routine clinical testings at SeekIn and Sun Yat-sen Memorial Hospital. 7565 participants (954 with cancer and 6611 without) from the two sites were divided into training and independent validation cohort. The second validation cohort (1005 with cancer and 812 without) was from Johns Hopkins University School of Medicine. Patients with cancer prior to therapy were eligible for inclusion in the study. Individuals with no history of cancer were enrolled from the participating sites as the non-cancer group. One tube of peripheral blood was collected from each participant and quantified a panel of seven selected protein tumour markers (PTMs) by a common clinical electrochemiluminescence immunoassay analyser. An algorithm named OncoSeek was established using artificial intelligence (AI) to distinguish patients with cancer from those without cancer by calculating the probability of cancer (POC) index based on the quantification results of the seven PTMs and clinical information including sex and age of the individuals and to predict the possible affected tissue of origin (TOO) for those who have been detected with cancer signals in blood.
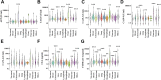
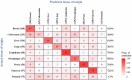
Findings: Between November 2012 and May 2022, 7565 participants were enrolled at SeekIn and Sun Yat-sen Memorial Hospital. The conventional clinical method, which relies only on a single threshold for each PTM, would suffer from a high false positive rate that accumulates as the number of markers increased. OncoSeek was empowered by AI technology to significantly reduce the false positive rate, increasing the specificity from 56.9% (95% confidence interval [CI]: 55.8-58.0) to 92.9% (92.3-93.5). In all cancer types, the overall sensitivity of OncoSeek was 51.7% (49.4-53.9), resulting in 84.3% (83.5-85.0) accuracy. The performance was generally consistent in the training and the two validation cohorts. The sensitivities ranged from 37.1% to 77.6% for the detection of the nine common cancer types (breast, colorectum, liver, lung, lymphoma, oesophagus, ovary, pancreas, and stomach), which account for ∼59.2% of global cancer deaths annually. Furthermore, it has shown excellent sensitivity in several high-mortality cancer types for which routine screening tests are lacking in the clinic, such as the sensitivity of pancreatic cancer which was 77.6% (69.3-84.6). The overall accuracy of TOO prediction in the true positives was 66.8%, which could assist the clinical diagnostic workup.
Interpretation: OncoSeek significantly outperforms the conventional clinical method, representing a novel blood-based test for MCED which is non-invasive, easy, efficient, and robust. Moreover, the accuracy of TOO facilitates the follow-up diagnostic workup.
Funding: The National Key Research and Development Programme of China.
Keywords: Artificial intelligence (AI); Liquid biopsy; Low- and middle-income countries (LMICs); Multi-cancer early detection (MCED); Protein tumour markers (PTMs).
© 2023 The Authors.
Conflict of interest statement
G.Z. is a full-time employee of and holds stock options in SeekIn Inc. S.L. is a full-time employee of and holds stock options in SeekIn Inc. W.W. is a full-time employee of and holds stock options in SeekIn Inc. Y.F. is a full-time employee of and holds stock options in SeekIn Inc. Y.Z. is a full-time employee of and holds stock options in SeekIn Inc. M.M. is a full-time employee of and holds stock options in SeekIn Inc. D.Z. is a full-time employee of Shenyou Bio, a wholly-owned subsidiary of SeekIn Inc, and holds stock options in SeekIn Inc. All other authors declare no competing interest.
Figures


Similar articles
-
Integrating Artificial Intelligence for Advancing Multiple-Cancer Early Detection via Serum Biomarkers: A Narrative Review.Cancers (Basel). 2024 Feb 21;16(5):862. doi: 10.3390/cancers16050862. Cancers (Basel). 2024. PMID: 38473224 Free PMC article. Review.
-
Highly sensitive detection platform-based diagnosis of oesophageal squamous cell carcinoma in China: a multicentre, case-control, diagnostic study.Lancet Digit Health. 2024 Oct;6(10):e705-e717. doi: 10.1016/S2589-7500(24)00153-5. Lancet Digit Health. 2024. PMID: 39332854
-
A Cost-Effective Two-Step Approach for Multi-Cancer Early Detection in High-Risk Populations.Cancer Res Commun. 2025 Jan 1;5(1):150-156. doi: 10.1158/2767-9764.CRC-24-0508. Cancer Res Commun. 2025. PMID: 39740056 Free PMC article.
-
Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study.Lancet Oncol. 2019 Dec;20(12):1645-1654. doi: 10.1016/S1470-2045(19)30637-0. Epub 2019 Oct 4. Lancet Oncol. 2019. PMID: 31591062 Clinical Trial.
-
Shifting the Cancer Screening Paradigm: Developing a Multi-Biomarker Class Approach to Multi-Cancer Early Detection Testing.Life (Basel). 2024 Jul 24;14(8):925. doi: 10.3390/life14080925. Life (Basel). 2024. PMID: 39202669 Free PMC article. Review.
Cited by
-
Integrating Artificial Intelligence for Advancing Multiple-Cancer Early Detection via Serum Biomarkers: A Narrative Review.Cancers (Basel). 2024 Feb 21;16(5):862. doi: 10.3390/cancers16050862. Cancers (Basel). 2024. PMID: 38473224 Free PMC article. Review.
-
AI-Derived Blood Biomarkers for Ovarian Cancer Diagnosis: Systematic Review and Meta-Analysis.J Med Internet Res. 2025 Mar 24;27:e67922. doi: 10.2196/67922. J Med Internet Res. 2025. PMID: 40126546 Free PMC article.
-
The Immune Landscape of Pheochromocytoma and Paraganglioma: Current Advances and Perspectives.Endocr Rev. 2024 Jul 12;45(4):521-552. doi: 10.1210/endrev/bnae005. Endocr Rev. 2024. PMID: 38377172 Free PMC article. Review.
-
Biomarkers in Cancer Screening: Promises and Challenges in Cancer Early Detection.Hematol Oncol Clin North Am. 2024 Aug;38(4):869-888. doi: 10.1016/j.hoc.2024.04.004. Epub 2024 May 22. Hematol Oncol Clin North Am. 2024. PMID: 38782647 Free PMC article. Review.
-
Novel biomarkers for monitoring and management of hepatocellular carcinoma.Cancer Cell Int. 2024 Dec 24;24(1):428. doi: 10.1186/s12935-024-03600-1. Cancer Cell Int. 2024. PMID: 39719624 Free PMC article. Review.
References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Mitchell E.P. Screening for lung cancer. J Natl Med Assoc. 2021;113:239–240. - PubMed
-
- Siu A.L. On behalf of the U.S. Preventive services task force. Screening for breast cancer: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2016;164:279. - PubMed
-
- US Preventive Services Task Force. Curry S.J., Krist A.H., et al. Screening for cervical cancer: US preventive services task force recommendation statement. JAMA. 2018;320:674. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

