Molecular determinants of TRPM8 function: key clues for a cool modulation
- PMID: 37388453
- PMCID: PMC10301734
- DOI: 10.3389/fphar.2023.1213337
Molecular determinants of TRPM8 function: key clues for a cool modulation
Abstract
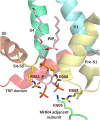
Cold thermoreceptor neurons detect temperature drops with highly sensitive molecular machinery concentrated in their peripheral free nerve endings. The main molecular entity responsible for cold transduction in these neurons is the thermo-TRP channel TRPM8. Cold, cooling compounds such as menthol, voltage, and osmolality rises activate this polymodal ion channel. Dysregulation of TRPM8 activity underlies several physiopathological conditions, including painful cold hypersensitivity in response to axonal damage, migraine, dry-eye disease, overactive bladder, and several forms of cancer. Although TRPM8 could be an attractive target for treating these highly prevalent diseases, there is still a need for potent and specific modulators potentially suitable for future clinical trials. This goal requires a complete understanding of the molecular determinants underlying TRPM8 activation by chemical and physical agonists, inhibition by antagonists, and the modulatory mechanisms behind its function to guide future and more successful treatment strategies. This review recapitulates information obtained from different mutagenesis approaches that have allowed the identification of specific amino acids in the cavity comprised of the S1-S4 and TRP domains that determine modulation by chemical ligands. In addition, we summarize different studies revealing specific regions within the N- and C-terminus and the transmembrane domain that contribute to cold-dependent TRPM8 gating. We also highlight the latest milestone in the field: cryo-electron microscopy structures of TRPM8, which have provided a better comprehension of the 21 years of extensive research in this ion channel, shedding light on the molecular bases underlying its modulation, and promoting the future rational design of novel drugs to selectively regulate abnormal TRPM8 activity under pathophysiological conditions.
Keywords: WS-12; cold; cryo-EM structures; icilin; ion channel; menthol.
Copyright © 2023 Pertusa, Solorza and Madrid.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Alarcón-Alarcón D., Cabañero D., de Andrés-López J., Nikolaeva-Koleva M., Giorgi S., Fernández-Ballester G., et al. (2022) TRPM8 contributes to sex dimorphism by promoting recovery of normal sensitivity in a mouse model of chronic migraine. Nat. Commun. 131 13:1–17. 10.1038/s41467-022-33835-3 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

