Enantio-Complementary Synthesis of 2-Substituted Pyrrolidines and Piperidines via Transaminase-Triggered Cyclizations
- PMID: 37388678
- PMCID: PMC10301811
- DOI: 10.1021/jacsau.3c00103
Enantio-Complementary Synthesis of 2-Substituted Pyrrolidines and Piperidines via Transaminase-Triggered Cyclizations
Abstract
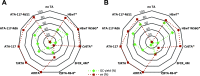
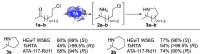
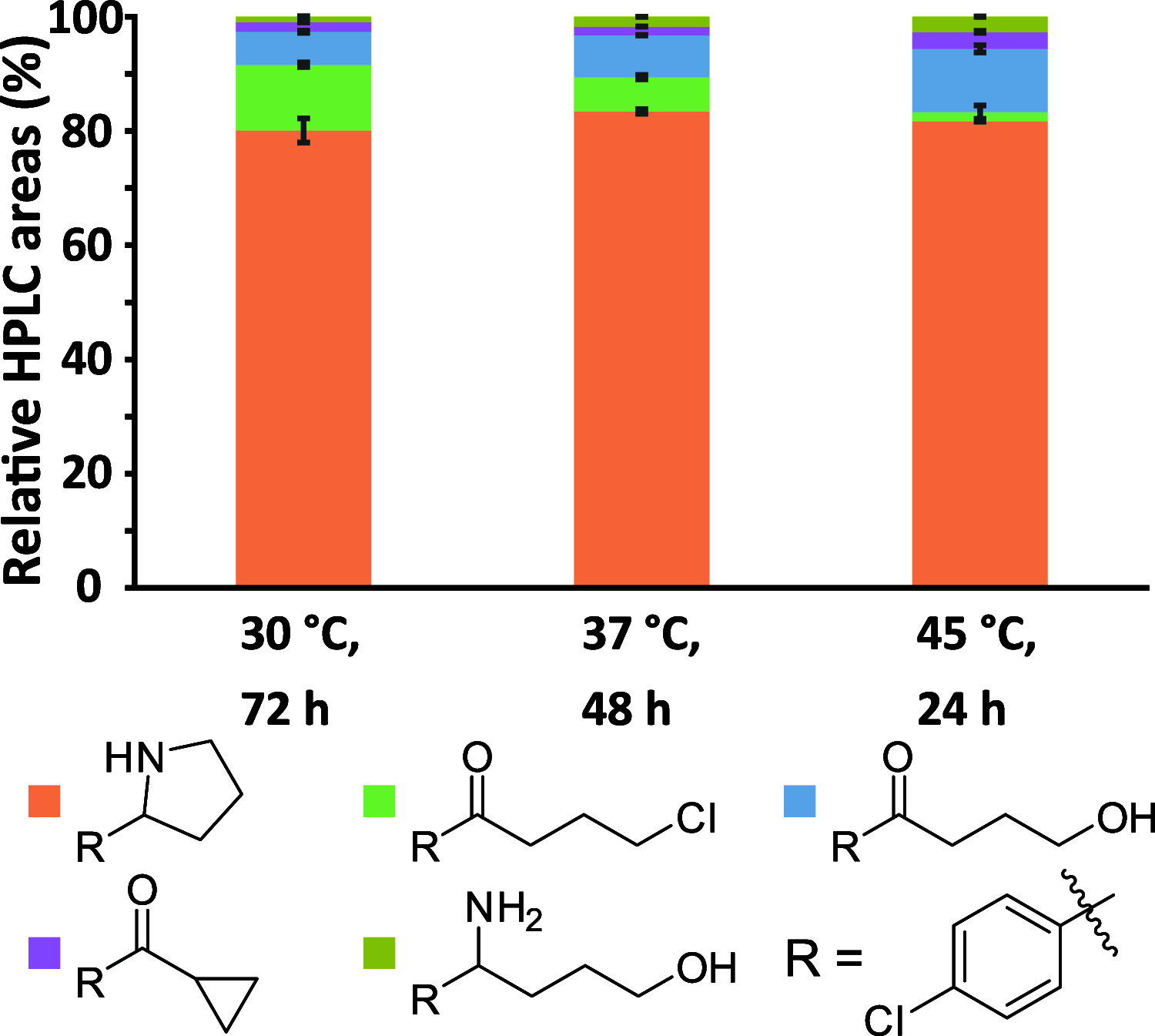
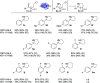
Chiral N-heterocycles are a common motif in many active pharmaceutical ingredients; however, their synthesis often relies on the use of heavy metals. In recent years, several biocatalytic approaches have emerged to reach enantiopurity. Here, we describe the asymmetric synthesis of 2-substituted pyrrolidines and piperidines, starting from commercially available ω-chloroketones by using transaminases, which has not yet been comprehensively studied. Analytical yields of up to 90% and enantiomeric excesses of up to >99.5% for each enantiomer were achieved, which has not previously been shown for bulky substituents. This biocatalytic approach was applied to synthesize (R)-2-(p-chlorophenyl)pyrrolidine on a 300 mg scale, affording 84% isolated yield, with >99.5% ee.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Ghislieri D.; Turner N. J. Biocatalytic Approaches to the Synthesis of Enantiomerically Pure Chiral Amines. Top. Catal. 2014, 57, 284–300. 10.1007/s11244-013-0184-1. - DOI
-
- Slabu I.; Galman J. L.; Lloyd R. C.; Turner N. J. Discovery, Engineering, and Synthetic Application of Transaminase Biocatalysts. ACS Catal. 2017, 7, 8263–8284. 10.1021/acscatal.7b02686. - DOI
LinkOut - more resources
Full Text Sources
