In Situ Multinuclear Magic-Angle Spinning NMR: Monitoring Crystallization of Molecular Sieve AlPO4-11 in Real Time
- PMID: 37388699
- PMCID: PMC10302754
- DOI: 10.1021/jacsau.3c00109
In Situ Multinuclear Magic-Angle Spinning NMR: Monitoring Crystallization of Molecular Sieve AlPO4-11 in Real Time
Abstract
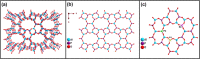
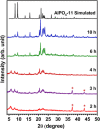
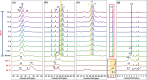
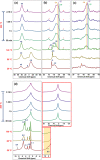
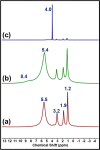
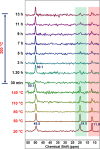
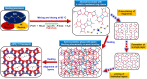
Molecular sieves are crystalline three-dimensional frameworks with well-defined channels and cavities. They have been widely used in industry for many applications such as gas separation/purification, ion exchange, and catalysis. Obviously, understanding the formation mechanisms is fundamentally important. High-resolution solid-state NMR spectroscopy is a powerful method for the study of molecular sieves. However, due to technical challenges, the vast majority of the high-resolution solid-state NMR studies on molecular sieve crystallization are ex situ. In the present work, using a new commercially available NMR rotor that can withhold high pressure and high temperature, we examined the formation of molecular sieve AlPO4-11 under dry gel conversion conditions by in situ multinuclear (1H, 27Al, 31P, and 13C) magic-angle spinning (MAS) solid-state NMR. In situ high-resolution NMR spectra obtained as a function of heating time provide much insights underlying the crystallization mechanism of AlPO4-11. Specifically, in situ 27Al and 31P MAS NMR along with 1H → 31P cross-polarization (CP) MAS NMR were used to monitor the evolution of the local environments of framework Al and P, in situ 1H → 13C CP MAS NMR to follow the behavior of the organic structure directing agent, and in situ 1H MAS NMR to unveil the effect of water content on crystallization kinetics. The in situ MAS NMR results lead to a better understanding of the formation of AlPO4-11.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Pool R. The Smallest Chemical Plants: Zeolites-Crystalline Materials Riddled with Nanometer-Sized Cavities-Can Exert Exquisite Control over Chemical Reactions and Produce Devices on the Smallest Scale. Science 1994, 263, 1698–1699. 10.1126/science.8134833. - DOI
-
- Wilson S. T.; Lok B. M.; Messina C. A.; Cannan T. R.; Flanigen E. M. Aluminophosphate Molecular Sieves: A New Class of Microporous Crystalline Inorganic Solids. J. Am. Chem. Soc. 1982, 104, 1146–1147. 10.1021/ja00368a062. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
