Proteomics of immune cells from liver tumors reveals immunotherapy targets
- PMID: 37388918
- PMCID: PMC10300607
- DOI: 10.1016/j.xgen.2023.100331
Proteomics of immune cells from liver tumors reveals immunotherapy targets
Abstract
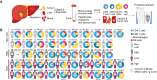
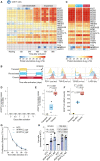
Elucidating the mechanisms by which immune cells become dysfunctional in tumors is critical to developing next-generation immunotherapies. We profiled proteomes of cancer tissue as well as monocyte/macrophages, CD4+ and CD8+ T cells, and NK cells isolated from tumors, liver, and blood of 48 patients with hepatocellular carcinoma. We found that tumor macrophages induce the sphingosine-1-phospate-degrading enzyme SGPL1, which dampened their inflammatory phenotype and anti-tumor function in vivo. We further discovered that the signaling scaffold protein AFAP1L2, typically only found in activated NK cells, is also upregulated in chronically stimulated CD8+ T cells in tumors. Ablation of AFAP1L2 in CD8+ T cells increased their viability upon repeated stimulation and enhanced their anti-tumor activity synergistically with PD-L1 blockade in mouse models. Our data reveal new targets for immunotherapy and provide a resource on immune cell proteomes in liver cancer.
Keywords: CRISPR in mouse T cells; HCC; NK cells; T cells; cancer immunotherapy; liver cancer; macrophages; mass spectrometry-based proteomics; profiles of tumor-infiltrating immune cells; proteomes.
© 2023 The Author(s).
Conflict of interest statement
The Geiger laboratory received funding from F. Hoffmann-La Roche AG for this study. M.F., T.N., H.M., and N.P. were or are employees and shareholders of F. Hoffmann-La Roche AG. R.G. is a co-founder of Encentrio Therapeutics and a member of the scientific executive board. L.T.J. is a co-founder and former board member of Cimeio Therapeutics AG.
Figures





References
-
- Perez-Riverol Y., Bai J., Bandla C., García-Seisdedos D., Hewapathirana S., Kamatchinathan S., Kundu D.J., Prakash A., Frericks-Zipper A., Eisenacher M., et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50:D543–D552. doi: 10.1093/nar/gkab1038. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

