A systems biology approach uncovers novel disease mechanisms in age-related macular degeneration
- PMID: 37388919
- PMCID: PMC10300496
- DOI: 10.1016/j.xgen.2023.100302
A systems biology approach uncovers novel disease mechanisms in age-related macular degeneration
Abstract
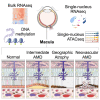
Age-related macular degeneration (AMD) is a leading cause of blindness, affecting 200 million people worldwide. To identify genes that could be targeted for treatment, we created a molecular atlas at different stages of AMD. Our resource is comprised of RNA sequencing (RNA-seq) and DNA methylation microarrays from bulk macular retinal pigment epithelium (RPE)/choroid of clinically phenotyped normal and AMD donor eyes (n = 85), single-nucleus RNA-seq (164,399 cells), and single-nucleus assay for transposase-accessible chromatin (ATAC)-seq (125,822 cells) from the retina, RPE, and choroid of 6 AMD and 7 control donors. We identified 23 genome-wide significant loci differentially methylated in AMD, over 1,000 differentially expressed genes across different disease stages, and an AMD Müller state distinct from normal or gliosis. Chromatin accessibility peaks in genome-wide association study (GWAS) loci revealed putative causal genes for AMD, including HTRA1 and C6orf223. Our systems biology approach uncovered molecular mechanisms underlying AMD, including regulators of WNT signaling, FRZB and TLE2, as mechanistic players in disease.
Keywords: AMD; DNA methylation; GA; Muller glia; RNA-seq; age-related macular degeneration; epigenomics; geographic atrophy; rare variant genetics; retinal pigment epithelium; single-cell ATAC-seq; single-cell RNA-seq.
© 2023 The Authors.
Conflict of interest statement
L.D.O., J.H., A.D.S., J.T., S.H., V.T.M., C.C., J.L., A. Sridhar, O.M., J.S.K., M.J.T., B.L.Y., and H.-H.C. are employees and shareholders of Genentech/Roche. I.K.K. is a consultant for Kodiak Sciences and Biophytis and receives research funding from Allergen. M.M.D. has a research grant from Genentech/Roche.
Figures





References
-
- Wong W.L., Su X., Li X., Cheung C.M.G., Klein R., Cheng C.-Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet. Glob. Health. 2014;2:e106–e116. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

