The SHELTER Trial of Transplanting Hepatitis C Virus-Infected Lungs Into Uninfected Recipients
- PMID: 37389016
- PMCID: PMC10306429
- DOI: 10.1097/TXD.0000000000001504
The SHELTER Trial of Transplanting Hepatitis C Virus-Infected Lungs Into Uninfected Recipients
Abstract
SHELTER is a trial of transplanting lungs from deceased donors with hepatitis C virus (HCV) infection into HCV-negative candidates (sponsor: Merck; NCT03724149). Few trials have reported outcomes using thoracic organs from HCV-RNA+ donors and none have reported quality of life (QOL).
Methods: This study is a single-arm trial of 10 lung transplants at a single center. Patients were included who were between 18 and 67 y of age and waitlisted for lung-only transplant. Patients were excluded who had evidence of liver disease. Primary outcome was HCV cure (sustained virologic response 12 wk after completing antiviral therapy). Recipients longitudinally reported QOL using the validated RAND-36 instrument. We also applied advanced methods to match HCV-RNA+ lung recipients to HCV-negative lung recipients in a 1:3 ratio at the same center.
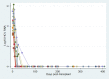
Results: Between November 2018 and November 2020, 18 patients were consented and opted-in for HCV-RNA+ lung offers in the allocation system. After a median of 37 d (interquartile range [IQR], 6-373) from opt-in, 10 participants received double lung transplants. The median recipient age was 57 y (IQR, 44-67), and 7 recipients (70%) had chronic obstructive pulmonary disease. The median lung allocation score at transplant was 34.3 (IQR, 32.7-86.9). Posttransplant, 5 recipients developed primary graft dysfunction grade 3 on day 2 or 3, although none required extracorporeal membrane oxygenation. Nine patients received elbasvir/grazoprevir, whereas 1 patient received sofosbuvir/velpatasvir. All 10 patients were cured of HCV and survived to 1 y (versus 83% 1-y survival among matched comparators). No serious adverse events were found to be related to HCV or treatment. RAND-36 scores showed substantial improvement in physical QOL and some improvement in mental QOL. We also examined forced expiratory volume in 1 s-the most important lung function parameter after transplantation. We detected no clinically important differences in forced expiratory volume in 1 s between the HCV-RNA+ lung recipients versus matched comparators.
Conclusions: SHELTER adds important evidence regarding the safety of transplanting HCV-RNA+ lungs into uninfected recipients and suggests QOL benefits.
Copyright © 2023 The Author(s). Transplantation Direct. Published by Wolters Kluwer Health, Inc.
Figures




References
-
- Valapour M, Lehr CJ, Skeans MA, et al. . OPTN/SRTR 2020 annual data report: lung. Am J Transplant. 2022;22 Suppl 2:438–518. - PubMed
-
- Phillips KG, Ranganath NK, Malas J, et al. . Impact of the opioid epidemic on heart transplantation: donor characteristics and organ discard. Ann Thorac Surg. 2019;108:1133–1139. - PubMed
-
- Reese PP, Abt PL, Blumberg EA, et al. . Twelve-month outcomes after transplant of hepatitis C-infected kidneys into uninfected recipients: a single-group trial. Ann Intern Med. 2018;169:273–281. - PubMed
-
- Sise ME, Goldberg DS, Kort JJ, et al. . Multicenter Study to Transplant Hepatitis C-Infected Kidneys (MYTHIC): an open-label study of combined glecaprevir and pibrentasvir to treat recipients of transplanted kidneys from deceased donors with hepatitis C virus infection. J Am Soc Nephrol. 2020;31:2678–2687. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

