Low-temperature synthesis, characterization and photocatalytic properties of lanthanum vanadate LaVO4
- PMID: 37389049
- PMCID: PMC10300335
- DOI: 10.1016/j.heliyon.2023.e17255
Low-temperature synthesis, characterization and photocatalytic properties of lanthanum vanadate LaVO4
Abstract
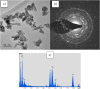
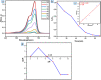
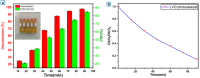
In this study, we have successfully prepared tetragonal lanthanum vanadate LaVO4 nanoparticles by a facile co-precipitation method at room temperature. The obtained materials were characterized using different structural and micro-structural techniques such as the characterization by X-ray diffraction (XRD), UV-Vis diffuse reflectance spectrum (DRS), transmission electron microscopy (TEM), and Raman spectrometry. The obtained structure is crystallized in single tetragonal phase with pin-like nanostructure. A main optical transition with bandgap energy of 3.26 eV is evidenced, and the average lifetime of charges carriers was found to be 1 ns Furthermore, the photoluminescence occurs in the visible light range. The photocatalytic activity was evaluated by the photocatalytic degradation of methylene blue (MB) with initial concentration of 10 mg L-1. The result indicates that LaVO4 particles showed a best photocatalytic activity of 98.2% degradation for methylene blue solution after irradiation of 90 min under visible light. Furthermore, the photocatalytic mechanism and reusability were studied.
Keywords: Dye degradation; Lanthanum vanadate; Optical properties; Photocatalysis.
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Figures






References
-
- Amjlef A., Khrach S., Ait El Fakir A., Farsad S., Et-Taleb S., El Alem N. Adsorptive properties investigation of natural sand as adsorbent for methylene blue removal from contaminated water. Nanotechnol. Environ. Eng. 2021;6:26. doi: 10.1007/s41204-021-00119-y. - DOI
-
- Saeid Taghavi Fardood A.N., Moradnia Farzaneh, Heidarzadeh Siamak. Green synthesis, characterization, photocatalytic and antibacterial activities of copper oxide nanoparticles of copper oxide nanoparticles. Nano Res. 2023;8:134–140. doi: 10.22036/ncr.2023.02.006. - DOI
-
- Li Z., Mei J., Bai L. Synthesis of C3N4-decorated ZnO and Ag/ZnO nanoparticles via calcination of ZIF-8 and melamine for photocatalytic removal of methyl orange. Chem. Pap. 2019;73:883–889. doi: 10.1007/s11696-018-0656-7. - DOI
-
- Akhsassi B., Bouddouch A., Naciri Y., Bakiz B., Taoufyq A., Favotto C., Villain S., Guinneton F., Benlhachemi A. Enhanced photocatalytic activity of Zn3(PO4)2/ZnO composite semiconductor prepared by different methods. Chem. Phys. Lett. 2021;783 doi: 10.1016/j.cplett.2021.139046. - DOI
-
- Shah L.A., Sayed M., Fayaz M., Bibi I., Nawaz M., Siddiq M. Ag-loaded thermo-sensitive composite microgels for enhanced catalytic reduction of methylene blue. Nanotechnol. Environ. Eng. 2017;2:14. doi: 10.1007/s41204-017-0026-7. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

