The epithelial barrier theory: Development and exacerbation of allergic and other chronic inflammatory diseases
- PMID: 37389096
- PMCID: PMC10166244
- DOI: 10.5415/apallergy.0000000000000005
The epithelial barrier theory: Development and exacerbation of allergic and other chronic inflammatory diseases
Abstract
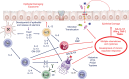
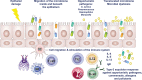
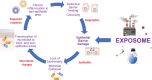
It is now longer than half a century, humans, animals, and nature of the world are under the influence of exposure to many newly introduced noxious substances. These exposures are nowadays pushing the borders to be considered as the causative or exacerbating factors for many chronic disorders including allergic, autoimmune/inflammatory, and metabolic diseases. The epithelial linings serve as the outermost body's primary physical, chemical, and immunological barriers against external stimuli. The "epithelial barrier theory" hypothesizes that these diseases are aggravated by an ongoing periepithelial inflammation triggered by exposure to a wide range of epithelial barrier-damaging insults that lead to "epithelitis" and the release of alarmins. A leaky epithelial barrier enables the microbiome's translocation from the periphery to interepithelial and even deeper subepithelial areas together with allergens, toxins, and pollutants. Thereafter, microbial dysbiosis, characterized by colonization of opportunistic pathogen bacteria and loss of the number and biodiversity of commensal bacteria take place. Local inflammation, impaired tissue regeneration, and remodeling characterize the disease. The infiltration of inflammatory cells to affected tissues shows an effort to expulse the tissue invading bacteria, allergens, toxins, and pollutants away from the deep tissues to the surface, representing the "expulsion response." Cells that migrate to other organs from the inflammatory foci may play roles in the exacerbation of various inflammatory diseases in distant organs. The purpose of this review is to highlight and appraise recent opinions and findings on epithelial physiology and its role in the pathogenesis of chronic diseases in view of the epithelial barrier theory.
Keywords: Allergy; asthma; autoimmune diseases; barrier dysfunction; epithelial barrier theory; epithelitis; microbiota; tight junctions.
Copyright © 2023. Asia Pacific Association of Allergy, Asthma and Clinical Immunology.
Conflict of interest statement
CAA has received research grants from the Swiss National Science Foundation, European Union (EU CURE, EU Syn-Air-G), Novartis Research Institutes, (Basel, Switzerland), Stanford University (Redwood City, CA), Seed Health (Boston, USA) and SciBase (Stockholm, Sweden); is the Co-Chair for EAACI Guidelines on Environmental Science in Allergic Diseases and Asthma; Chair of the EAACI Epithelial Cell Biology Working Group; is on the Advisory Boards of Sanofi/Regeneron (Bern, Switzerland; New York, USA), Stanford University Sean Parker Asthma Allergy Center (CA, USA), Novartis (Basel, Switzerland), Glaxo Smith Kline (Zurich, Switzerland), Bristol-Myers Squibb (New York, USA), Seed Health (Boston, USA), and SciBase (Stockholm, Sweden); and is the Editor-in-Chief of Allergy. MA has received research grants from Swiss National Science Foundation, Bern; research grant from the Stanford University; Leading House for the Latin American Region, Seed Money Grant. She is in the Scientific Advisory Board member of Stanford University Sean Parker Asthma Allergy Center (CA, USA); Advisory Board member of LEO Foundation Skin Immunology Research Center (Kopenhagen, Denmark); and Scientific Co-Chair of World Allergy Congress (WAC) Istanbul, 2022, Scientific Programme Committee Chair, EAACI. KN is the Director of the World Allergy Organization Center of Excellence for Stanford, Advisor at Cour Pharma, Consultant for Excellergy, Red tree ventures, Eli Lilly, and Phylaxis, Co-founder of Before Brands, Alladapt, Latitude, and IgGenix; and National Scientific Committee member at Immune Tolerance Network (ITN), and National Institutes of Health (NIH) clinical research centers, outside the submitted work; patents include “Mixed allergen composition and methods for using the same,” “Granulocyte-based methods for detecting and monitoring immune system disorders,” and “Methods and Assays for Detecting and Quantifying Pure Subpopulations of White Blood Cells in Immune System Disorders.” The remaining authors declare no conflicts of interest.
Figures

References
-
- Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions?. Nat Rev Immunol 2021;21:739–751. - PubMed
-
- Trautmann A, Schmid-Grendelmeier P, Kruger K, Crameri R, Akdis M, Akkaya A, Bröcker E-B, Blaser K, Akdis CA. T cells and eosinophils cooperate in the induction of bronchial epithelial cell apoptosis in asthma. J Allergy Clin Immunol 2002;109:329–37. - PubMed
-
- Kraehenbuhl JP, Pringault E, Neutra MR. Review article: intestinal epithelia and barrier functions. Aliment Pharmacol Ther. 1997;11 Suppl 3:3–8; discussion 8; discussion -9. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
