Neurological outcomes in immune checkpoint inhibitor-related neurotoxicity
- PMID: 37389303
- PMCID: PMC10306160
- DOI: 10.1093/braincomms/fcad169
Neurological outcomes in immune checkpoint inhibitor-related neurotoxicity
Abstract
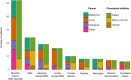
While the spectrum of neurological immune checkpoint inhibitor-related adverse events is expanding, patients' outcomes are not well documented. This study aimed to assess outcomes of neurological immune-related adverse events and to identify prognostic factors. All patients experiencing grade ≥2 neurological immune-related adverse events identified at two clinical networks (French Reference Center for Paraneoplastic Neurological Syndromes, Lyon; and OncoNeuroTox, Paris) over five years were included. Modified Rankin scores were assessed at onset, 6, 12, 18 months, and last visit. A multi-state Markov model was used to estimate the transition rates between minor disability (mRS <3), severe disability (mRS 3-5), and death (mRS 6), over the study period. The state-to-state transition rates were estimated using maximum likelihood and variables were introduced into the different transitions to study their effects. A total of 147 patients were included out of 205 patients with a suspicion of neurological immune-related adverse events. The median age was 65 years (range 20-87) and 87/147 patients (59.2%) were male. Neurological immune-related adverse events involved the peripheral nervous system in 87/147 patients (59.2%), the central nervous system in 51/147 (34.7%), and both systems in 9/147 (6.1%). Paraneoplastic-like syndromes were observed in 30/147 patients (20.4%). Cancers included lung cancers (36.1%), melanoma (30.6%), urological cancers (15.6%), and others (17.8%). Patients were treated with programmed cell death protein (ligan) 1 (PD(L)1) inhibitors (70.1%), CTLA4 inhibitors (3.4%) or both (25.9%). Severe disability was reported in 108/144 patients (75.0%) at onset and in 33/146 patients (22.6%) at last visit (median follow-up duration: 12 months, range 0.5-50); 48/147 (32.7%) patients died, from cancer progression (17/48, 35.4%), neurological toxicity (15/48, 31.2%), other causes (10/48, 20.8%) or unknown causes (6/48, 12.5%). The rate of transition from severe to minor disability independently increased with melanoma [compared to lung cancer, hazard ratio = 3.26, 95%CI (1.27; 8.41)] and myositis/neuromuscular junction disorders [hazard ratio = 8.26, 95%CI (2.90; 23.58)], and decreased with older age [hazard ratio = 0.68, 95%CI (0.47; 0.99)] and paraneoplastic-like syndromes [hazard ratio = 0.29, 95%CI (0.09; 0.98)]. In patients with neurological immune-related adverse events, myositis/neuromuscular junction disorders and melanoma increase the transition rate from severe to minor disability, while older age and paraneoplastic-like syndromes result in poorer neurological outcomes; future studies are needed to optimize the management of such patients.
Keywords: cancer immunotherapy complications; immune checkpoint inhibitors; neurological immune-related adverse events; neurotoxicity; paraneoplastic neurological syndromes.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures




References
-
- Martins F, Sykiotis GP, Maillard M, et al. . New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. 2019;20(1):e54–e64. - PubMed
-
- Sato K, Mano T, Iwata A, Toda T. Neurological and related adverse events in immune checkpoint inhibitors: A pharmacovigilance study from the Japanese adverse drug event report database. J Neurooncol. 2019;145(1):1–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
