Cerebrospinal fluid biomarkers for cerebral amyloid angiopathy
- PMID: 37389304
- PMCID: PMC10300526
- DOI: 10.1093/braincomms/fcad159
Cerebrospinal fluid biomarkers for cerebral amyloid angiopathy
Abstract
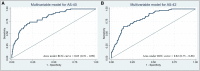
Integrating cerebrospinal fluid-biomarkers into diagnostic workup of patients with sporadic cerebral amyloid angiopathy may support early and correct identification. We aimed to identify and validate clinical- and cerebrospinal fluid-biomarkers for in vivo diagnosis of cerebral amyloid angiopathy. This observational cohort study screened 2795 consecutive patients admitted for cognitive complaints to the academic departments of neurology and psychiatry over a 10-year period (2009-2018). We included 372 patients with available hemosiderin-sensitive MR imaging and cerebrospinal fluid-based neurochemical dementia diagnostics, i.e. Aβ40, Aβ42, t-tau, p-tau. We investigated the association of clinical- and cerebrospinal fluid-biomarkers with the MRI-based diagnosis of cerebral amyloid angiopathy, applying confounder-adjusted modelling, receiver operating characteristic and unsupervised cluster analyses. We identified 67 patients with cerebral amyloid angiopathy, 76 patients with Alzheimer's disease, 75 patients with mild cognitive impairment due to Alzheimer's disease, 76 patients with mild cognitive impairment with unlikely Alzheimer's disease and 78 healthy controls. Patients with cerebral amyloid angiopathy showed a specific cerebrospinal fluid pattern: average concentration of Aß40 [13 792 pg/ml (10 081-18 063)] was decreased compared to all controls (P < 0.05); Aß42 [634 pg/ml (492-834)] was comparable to Alzheimer's disease and mild cognitive impairment due to Alzheimer's disease (P = 0.10, P = 0.93) but decreased compared to mild cognitive impairment and healthy controls (both P < 0.001); p-tau [67.3 pg/ml (42.9-91.9)] and t-tau [468 pg/ml (275-698)] were decreased compared to Alzheimer's disease (P < 0.001, P = 0.001) and mild cognitive impairment due to Alzheimer's disease (P = 0.001, P = 0.07), but elevated compared to mild cognitive impairment and healthy controls (both P < 0.001). Multivariate modelling validated independent clinical association of cerebral amyloid angiopathy with older age [odds-ratio: 1.06, 95% confidence interval (1.02-1.10), P < 0.01], prior lobar intracerebral haemorrhage [14.00 (2.64-74.19), P < 0.01], prior ischaemic stroke [3.36 (1.58-7.11), P < 0.01], transient focal neurologic episodes (TFNEs) [4.19 (1.06-16.64), P = 0.04] and gait disturbance [2.82 (1.11-7.15), P = 0.03]. For cerebrospinal fluid-biomarkers per 1 pg/ml, both lower Aß40 [0.9999 (0.9998-1.0000), P < 0.01] and lower Aß42 levels [0.9989 (0.9980-0.9998), P = 0.01] provided an independent association with cerebral amyloid angiopathy controlled for all aforementioned clinical confounders. Both amyloid biomarkers showed good discrimination for diagnosis of cerebral amyloid angiopathy among adjusted receiver operating characteristic analyses (area under the receiver operating characteristic curves, Aß40: 0.80 (0.73-0.86), P < 0.001; Aß42: 0.81 (0.75-0.88), P < 0.001). Unsupervised Euclidian clustering of all cerebrospinal fluid-biomarker-profiles resulted in distinct segregation of cerebral amyloid angiopathy patients from all controls. Together, we demonstrate that a distinctive set of cerebrospinal fluid-biomarkers effectively differentiate cerebral amyloid angiopathy patients from patients with Alzheimer's disease, mild cognitive impairment with or without underlying Alzheimer's disease, and healthy controls. Integrating our findings into a multiparametric approach may facilitate diagnosing cerebral amyloid angiopathy, and may aid clinical decision-making, but warrants future prospective validation.
Keywords: biomarker; cerebral amyloid angiopathy; cerebrospinal fluid; dementia; small vessel disease.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
M.I.S. reports grants from the Interdisciplinary Center for Clinical Research and Marohn Foundation. P.L. received consultation and/or lecture honoraria from IBL International, Fujirebio Europe, AJ Roboscreen, Biogen, and Roche. J.B.K. received speaker honoraria from Sanofi, Biogen, Bayer, Alexion, Boston Scientific, and BI. The other authors report no conflicts of interest related to the contents of the manuscript.
Figures





Similar articles
-
Comparative methods for quantifying plasma biomarkers in Alzheimer's disease: Implications for the next frontier in cerebral amyloid angiopathy diagnostics.Alzheimers Dement. 2024 Feb;20(2):1436-1458. doi: 10.1002/alz.13510. Epub 2023 Oct 31. Alzheimers Dement. 2024. PMID: 37908054 Free PMC article. Review.
-
Characterizing mixed location hemorrhages/microbleeds with CSF markers.Int J Stroke. 2023 Jul;18(6):728-735. doi: 10.1177/17474930231152124. Epub 2023 Feb 1. Int J Stroke. 2023. PMID: 36622021
-
A data-driven model of biomarker changes in sporadic Alzheimer's disease.Brain. 2014 Sep;137(Pt 9):2564-77. doi: 10.1093/brain/awu176. Epub 2014 Jul 9. Brain. 2014. PMID: 25012224 Free PMC article.
-
Cerebral microbleeds topography and cerebrospinal fluid biomarkers in cognitive impairment.J Cereb Blood Flow Metab. 2017 Mar;37(3):1006-1013. doi: 10.1177/0271678X16649401. Epub 2016 Jul 21. J Cereb Blood Flow Metab. 2017. PMID: 27178426 Free PMC article.
-
Cerebrospinal fluid biomarkers and apolipoprotein E genotype in cerebral amyloid angiopathy. A narrative review.Cereb Circ Cogn Behav. 2021 Mar 21;2:100010. doi: 10.1016/j.cccb.2021.100010. eCollection 2021. Cereb Circ Cogn Behav. 2021. PMID: 36324707 Free PMC article. Review.
Cited by
-
CSF and plasma biomarkers in cerebral amyloid angiopathy: A single-center study and a systematic review/meta-analysis.Eur Stroke J. 2025 Mar;10(1):278-288. doi: 10.1177/23969873241260538. Epub 2024 Jun 13. Eur Stroke J. 2025. PMID: 38869035 Free PMC article.
-
Cerebral Amyloid Angiopathy in Patients with Cognitive Impairment: Cerebrospinal Fluid Biomarkers.Dement Geriatr Cogn Disord. 2024;53(5):248-254. doi: 10.1159/000539884. Epub 2024 Jun 18. Dement Geriatr Cogn Disord. 2024. PMID: 38889704 Free PMC article.
-
Editorial: Cerebral amyloid angiopathy: from bench to bedside.Front Neurosci. 2024 Feb 6;18:1370352. doi: 10.3389/fnins.2024.1370352. eCollection 2024. Front Neurosci. 2024. PMID: 38379758 Free PMC article. No abstract available.
-
Plasma Biomarkers for Cerebral Amyloid Angiopathy and Implications for Amyloid-Related Imaging Abnormalities: A Comprehensive Review.J Clin Med. 2025 Feb 7;14(4):1070. doi: 10.3390/jcm14041070. J Clin Med. 2025. PMID: 40004604 Free PMC article. Review.
-
Comparative methods for quantifying plasma biomarkers in Alzheimer's disease: Implications for the next frontier in cerebral amyloid angiopathy diagnostics.Alzheimers Dement. 2024 Feb;20(2):1436-1458. doi: 10.1002/alz.13510. Epub 2023 Oct 31. Alzheimers Dement. 2024. PMID: 37908054 Free PMC article. Review.
References
-
- Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: Recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry. 2012;83(2):124–137. - PubMed
-
- Ter Telgte A, van Leijsen EMC, Wiegertjes K, Klijn CJM, Tuladhar AM, de Leeuw FE. Cerebral small vessel disease: From a focal to a global perspective. Nat Rev Neurol. 2018;14(7):387–398. - PubMed
LinkOut - more resources
Full Text Sources
