Magnesium Anode Protection by an Organic Artificial Solid Electrolyte Interphase for Magnesium-Sulfur Batteries
- PMID: 37389477
- PMCID: PMC10347424
- DOI: 10.1021/acsami.3c07223
Magnesium Anode Protection by an Organic Artificial Solid Electrolyte Interphase for Magnesium-Sulfur Batteries
Abstract
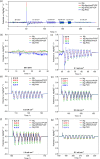
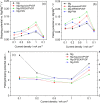
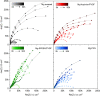
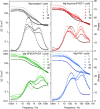
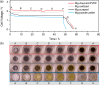
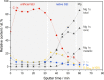
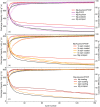
In the search for post-lithium battery systems, magnesium-sulfur batteries have attracted research attention in recent years due to their high potential energy density, raw material abundance, and low cost. Despite significant progress, the system still lacks cycling stability mainly associated with the ongoing parasitic reduction of sulfur at the anode surface, resulting in the loss of active materials and passivating surface layer formation on the anode. In addition to sulfur retention approaches on the cathode side, the protection of the reductive anode surface by an artificial solid electrolyte interphase (SEI) represents a promising approach, which contrarily does not impede the sulfur cathode kinetics. In this study, an organic coating approach based on ionomers and polymers is pursued to combine the desired properties of mechanical flexibility and high ionic conductivity while enabling a facile and energy-efficient preparation. Despite exhibiting higher polarization overpotentials in Mg-Mg cells, the charge overpotential in Mg-S cells was decreased by the coated anodes with the initial Coulombic efficiency being significantly increased. Consequently, the discharge capacity after 300 cycles applying an Aquivion/PVDF-coated Mg anode was twice that of a pristine Mg anode, indicating effective polysulfide repulsion from the Mg surface by the artificial SEI. This was backed by operando imaging during long-term OCV revealing a non-colored separator, i.e. mitigated self-discharge. While SEM, AFM, IR and XPS were applied to gain further insights into the surface morphology and composition, scalable coating techniques were investigated in addition to ensure practical relevance. Remarkably therein, the Mg anode preparation and all surface coatings were prepared under ambient conditions, which facilitates future electrode and cell assembly. Overall, this study highlights the important role of Mg anode coatings to improve the electrochemical performance of magnesium-sulfur batteries.
Keywords: artificial solid electrolyte interphase; coating techniques; electrochemical impedance spectroscopy; ionomers; magnesium anode; magnesium−sulfur battery.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Ford H. O.; Doyle E.; He P.; Boggess W. C.; Oliver A. G.; Wu T.; Sterbinsky G. E.; Schaefer J. Self-Discharge of Magnesium–Sulfur Batteries Leads to Active Material Loss and Poor Shelf Life. Energy Environ. Sci. 2021, 14, 890–899. 10.1039/D0EE01578D. - DOI
-
- Häcker J.; Nguyen D. H.; Rommel T.; Zhao-Karger Z.; Wagner N.; Friedrich K. A. Operando UV/Vis Spectroscopy providing Insights into the Sulfur and Polysulfide Dissolution in Magnesium-Sulfur Batteries. ACS Energy Lett. 2022, 7, 1–9. 10.1021/acsenergylett.1c02152. - DOI
-
- Häcker J.; Danner C.; Sievert B.; Biswas I.; Zhao-Karger Z.; Wagner N.; Friedrich K. A. Investigation of Magnesium–Sulfur Batteries using Electrochemical Impedance Spectroscopy. Electrochim. Acta 2020, 338, 135787 10.1016/j.electacta.2020.135787. - DOI
-
- Sievert B.; Häcker J.; Bienen F.; Wagner N.; Friedrich K. A. Magnesium Sulfur Battery with a New Magnesium Powder Anode. ECS Trans. 2017, 77, 413 10.1149/07711.0413ecst. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

