Towards Characterization of Skin Melanoma in the Clinic by Electron Paramagnetic Resonance (EPR) Spectroscopy and Imaging of Melanin
- PMID: 37389709
- PMCID: PMC11211150
- DOI: 10.1007/s11307-023-01836-3
Towards Characterization of Skin Melanoma in the Clinic by Electron Paramagnetic Resonance (EPR) Spectroscopy and Imaging of Melanin
Abstract
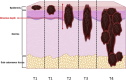
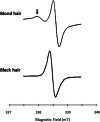
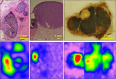
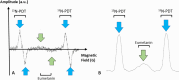
The incidence of melanoma is continuously increasing over time. Melanoma is the most aggressive skin cancer, significantly reducing quality of life and survival rates of patients at advanced stages. Therefore, early diagnosis remains the key to change the prognosis of patients with melanoma. In this context, advanced technologies are under evaluation to increase the accuracy of the diagnostic, to better characterize the lesions and visualize their possible invasiveness in the epidermis. Among the innovative methods, because melanin is paramagnetic, clinical low frequency electron paramagnetic resonance (EPR) that characterizes the melanin content in the lesion has the potential to be an adjunct diagnostic method of melanoma. In this review, we first summarize the challenges faced by dermatologists and oncologists in melanoma diagnostic and management. We also provide a historical perspective on melanin detection with a focus on EPR spectroscopy/imaging of melanomas. We describe key elements that allow EPR to move from in vitro studies to in vivo and finally to patients for melanoma studies. Finally, we provide a critical view on challenges to meet to make EPR operational in the clinic to characterize pigmented lesions.
Keywords: In vivo; Cancer; Clinical EPR; EPR; ESR; Low frequency; Melanin; Melanoma; Mice; Patients.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Halpern AC, Marghoob AA, Sober AJ, Mar V, Marchetti MA, et al. Clinical presentations of melanoma. In: Balch CM, et al., editors. Cutaneous Melanoma. 6. Springer; 2020. pp. 108–145.
-
- Piccolo D, Ferrari A, Peris K, Daidone R, Ruggeri B, Chimenti S. Dermoscopic diagnosis by a trained clinician vs. a clinician with minimal dermoscopy training vs. computer-aided diagnosis of 341 pigmented skin lesions: A comparative study. Br J Dermatol. 2002;147(3):481–486. doi: 10.1046/j.1365-2133.2002.04978.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

