Safety, tolerability, and immunogenicity of a 21-valent pneumococcal conjugate vaccine, V116, in Japanese healthy adults: A Phase I study
- PMID: 37389808
- PMCID: PMC10316726
- DOI: 10.1080/21645515.2023.2228162
Safety, tolerability, and immunogenicity of a 21-valent pneumococcal conjugate vaccine, V116, in Japanese healthy adults: A Phase I study
Abstract
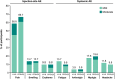
V116 is an investigational 21-valent pneumococcal conjugate vaccine (PCV) to address the burden of residual adult pneumococcal disease after the introduction of pediatric PCVs into national immunization programs (NIPs) and includes serotypes highly prevalent in adult invasive pneumococcal disease (IPD). This Phase I study assessed the safety, tolerability, and immunogenicity of V116 in Japanese adults. Participants ≥20 years of age were randomized to receive a single dose of V116 or 23-valent pneumococcal polysaccharide vaccine (PPSV23) at day 1. Outcomes were solicited injection-site and systemic adverse events (AEs) from day 1 to day 5, vaccine-related serious AEs from day 1 through day 30, and serotype-specific opsonophagocytic antibody (OPA) titers and immunoglobulin G (IgG) concentrations at day 30. Overall, 102 participants were randomized 1:1 to each group. Comparable proportions vaccinated with V116 and PPSV23 experienced ≥1 solicited injection-site AE and ≥1 solicited systemic AE. The most common injection-site AEs were injection-site pain (V116: 54.9%; PPSV23: 66.7%) and swelling (V116 and PPSV23: 13.7%), and the most common systemic AEs were myalgia (V116: 17.6%; PPSV23: 19.6%) and fatigue (V116: 13.7%; PPSV23: 9.8%). Solicited AEs were mostly mild and of ≤3 days duration. No vaccine-related serious AEs or deaths were reported. The OPA and IgG findings showed that the immunogenicity of V116 and PPSV23 were comparable for the 12 common serotypes and V116 was more immunogenic for the nine unique serotypes compared with PPSV23. V116 was well tolerated, with a safety profile similar to PPSV23, and induced functional antibodies against all 21 serotypes.
Keywords: 21-valent; Japanese; V116; adults; immunogenicity; pneumococcal conjugate vaccine; pneumococcal disease; safety; tolerability.
Conflict of interest statement
H.P. is an employee of Merck & Co., Inc., Rahway, NJ, USA and may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA. H.K., N.O., M.S., R.I. are employees of MSD K.K., Tokyo, Japan and may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA. M.H. and M.Y. have no conflicts of interest to report.
Figures

References
-
- Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1459–8. doi:10.1016/S0140-6736(16)31012-1. - DOI - PMC - PubMed
-
- Yanagihara K, Kosai K, Mikamo H, Mukae H, Takesue Y, Abe M, Taniguchi K, Petigara T, Kaku M.. Serotype distribution and antimicrobial susceptibility of Streptococcus pneumoniae associated with invasive pneumococcal disease among adults in Japan. Int J Infect Dis. 2021;102:260–8. doi:10.1016/j.ijid.2020.10.017. - DOI - PubMed
-
- Fukusumi M, Chang B, Tanabe Y, Oshima K, Maruyama T, Watanabe H, Kuronuma K, Kasahara K, Takeda H, Nishi J, et al. Invasive pneumococcal disease among adults in Japan, April 2013 to March 2015: disease characteristics and serotype distribution. BMC Infect Dis. 2017;17(1):2. doi:10.1186/s12879-016-2113-y. - DOI - PMC - PubMed
-
- Morimoto K, Suzuki M, Ishifuji T, Yaegashi M, Asoh N, Hamashige N, Abe M, Aoshima M, Ariyoshi K, Adult Pneumonia Study Group - Japan (APSG-J). The burden and etiology of community-onset pneumonia in the aging Japanese population: a multicenter prospective study. PLoS One. 2015;10(3):e0122247. doi:10.1371/journal.pone.0122247. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
