Introducing a new type of alternative laryngeal mucosa model
- PMID: 37390090
- PMCID: PMC10313048
- DOI: 10.1371/journal.pone.0287634
Introducing a new type of alternative laryngeal mucosa model
Abstract
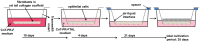
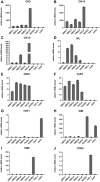
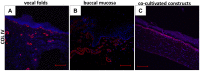
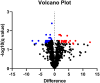
Research of human vocal fold (VF) biology is hampered by several factors. The sensitive microstructure of the VF mucosa is one of them and limits the in vivo research, as biopsies carry a very high risk of scarring. A laryngeal organotypic model consisting of VF epithelial cells and VF fibroblasts (VFF) may overcome some of these limitations. In contrast to human VFF, which are available in several forms, availability of VF epithelial cells is scarce. Buccal mucosa might be a good alternative source for epithelial cells, as it is easily accessible, and biopsies heal without scarring. For this project, we thus generated alternative constructs consisting of immortalized human VF fibroblasts and primary human buccal epithelial cells. The constructs (n = 3) were compared to native laryngeal mucosa at the histological and proteomic level. The engineered constructs reassembled into a mucosa-like structure after a cultivation period of 35 days. Immunohistochemical staining confirmed a multi-layered stratified epithelium, a collagen type IV positive barrier-like structure resembling the basement membrane, and an underlying layer containing VFF. Proteomic analysis resulted in a total number of 1961 identified and quantified proteins. Of these, 83.8% were detected in both native VF and constructs, with only 53 proteins having significantly different abundance. 15.3% of detected proteins were identified in native VF mucosa only, most likely due to endothelial, immune and muscle cells within the VF samples, while 0.9% were found only in the constructs. Based on easily available cell sources, we demonstrate that our laryngeal mucosa model shares many characteristics with native VF mucosa. It provides an alternative and reproducible in vitro model and offers many research opportunities ranging from the study of VF biology to the testing of interventions (e.g. drug testing).
Copyright: © 2023 Grossmann et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

