How I treat pediatric venous thromboembolism in the DOAC era
- PMID: 37390311
- PMCID: PMC10862368
- DOI: 10.1182/blood.2022018966
How I treat pediatric venous thromboembolism in the DOAC era
Abstract
The direct oral anticoagulants (DOACs) rivaroxaban and dabigatran are newly licensed for the treatment and prevention of venous thromboembolism (VTE) in children and mark a renaissance in pediatric anticoagulation management. They provide a convenient option over standard-of-care anticoagulants (heparins, fondaparinux, and vitamin K antagonists) because of their oral route of administration, child-friendly formulations, and significant reduction in monitoring. However, limitations related to therapeutic monitoring when needed and the lack of approved reversal agents for DOACs in children raise some safety concerns. There is accumulating experience of safety and efficacy of DOACs in adults for a broad scope of indications; however, the cumulative experience of using DOACs in pediatrics, specifically for those with coexisting chronic illnesses, is sparse. Consequently, clinicians must often rely on their experience for treating VTE and extrapolate from data in adults while using DOACs in children. In this article, the authors share their experience of managing 4 scenarios that hematologists are likely to encounter in their day-to-day practice. Topics addressed include (1) appropriateness of indication; (2) use for special populations of children; (3) considerations for laboratory monitoring; (4) transition between anticoagulants; (5) major drug interactions; (6) perioperative management; and (7) anticoagulation reversal.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: R.V.B. reports being an institutional principal investigator for pediatric VTE trials funded by Pfizer and Bayer and receiving an investigator-initiated grant from the Bristol Myers Squibb–Pfizer alliance to study risk factors of neonatal thrombosis. G.Y. was an institutional principal investigator for pediatric VTE trials funded by Bayer and Daiichi-Sankyo; has received consulting fees and honoraria from Bayer and Daiichi-Sankyo; and received a licensing fee from Viatris. A.A.S. was an institutional principal investigator for pediatric VTE trials funded by the National Institutes of Health, Pfizer, and Boehringer-Ingelheim.
Figures

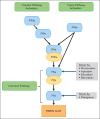
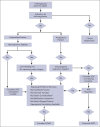


References
-
- Roehrig S, Straub A, Pohlmann J, et al. Discovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3- [4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene- 2-carboxamide (BAY 59-7939): an oral, direct factor Xa inhibitor. J Med Chem. 2005;48(19):5900–5908. - PubMed
-
- Branstetter JW, Kiskaddon AL, King MA, et al. Efficacy and safety of non-vitamin K antagonist oral anticoagulants in pediatric venous thromboembolism treatment and thromboprophylaxis: a systematic review of the literature. Semin Thromb Hemost. 2021;47(6):643–653. - PubMed
-
- Di Nisio M, Middeldorp S, Büller HR. Direct thrombin inhibitors. N Engl J Med. 2005;353(10):1028–1040. - PubMed
-
- Harder S, Graff J. Novel oral anticoagulants: clinical pharmacology, indications and practical considerations. Eur J Clin Pharmacol. 2013;69(9):1617–1633. - PubMed
-
- Laux V, Perzborn E, Heitmeier S, et al. Direct inhibitors of coagulation proteins - the end of the heparin and low-molecular-weight heparin era for anticoagulant therapy? Thromb Haemost. 2009;102(5):892–899. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

