Proof-of-concept and Randomized, Placebo-controlled Trials of an FcRn Inhibitor, Batoclimab, for Thyroid Eye Disease
- PMID: 37390454
- PMCID: PMC10655547
- DOI: 10.1210/clinem/dgad381
Proof-of-concept and Randomized, Placebo-controlled Trials of an FcRn Inhibitor, Batoclimab, for Thyroid Eye Disease
Abstract
Context: Inhibition of the neonatal fragment crystallizable receptor (FcRn) reduces pathogenic thyrotropin receptor antibodies (TSH-R-Ab) that drive pathology in thyroid eye disease (TED).
Objective: We report the first clinical studies of an FcRn inhibitor, batoclimab, in TED.
Design: Proof-of-concept (POC) and randomized, double-blind placebo-controlled trials.
Setting: Multicenter.
Participants: Patients with moderate-to-severe, active TED.
Intervention: In the POC trial, patients received weekly subcutaneous injections of batoclimab 680 mg for 2 weeks, followed by 340 mg for 4 weeks. In the double-blind trial, patients were randomized 2:2:1:2 to weekly batoclimab (680 mg, 340 mg, 255 mg) or placebo for 12 weeks.
Main outcome: Change from baseline in serum anti-TSH-R-Ab and total IgG (POC); 12-week proptosis response (randomized trial).
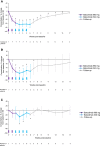
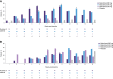
Results: The randomized trial was terminated because of an unanticipated increase in serum cholesterol; therefore, data from 65 of the planned 77 patients were analyzed. Both trials showed marked decreases in pathogenic anti-TSH-R-Ab and total IgG serum levels (P < .001) with batoclimab. In the randomized trial, there was no statistically significant difference with batoclimab vs placebo in proptosis response at 12 weeks, although significant differences were observed at several earlier timepoints. In addition, orbital muscle volume decreased (P < .03) at 12 weeks, whereas quality of life (appearance subscale) improved (P < .03) at 19 weeks in the 680-mg group. Batoclimab was generally well tolerated, with albumin reductions and increases in lipids that reversed upon discontinuation.
Conclusions: These results provide insight into the efficacy and safety of batoclimab and support its further investigation as a potential therapy for TED.
Keywords: FcRn; batoclimab; immunoglobulin G; neonatal fragment crystallizable receptor; thyroid eye disease; thyrotropin receptor autoantibodies.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Figures




Comment in
-
The Race for New Treatments for Graves Orbitopathy (Thyroid Eye Disease).J Clin Endocrinol Metab. 2024 Sep 16;109(10):e1938-e1939. doi: 10.1210/clinem/dgad693. J Clin Endocrinol Metab. 2024. PMID: 38451776 No abstract available.
References
-
- Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves’ Orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185(4):G43‐G67. - PubMed
-
- Davies TF, Andersen S, Latif R, et al. Graves’ disease. Nat Rev Dis Primers. 2020;6(1):52. - PubMed
-
- Taylor PN, Zhang L, Lee RWJ, et al. New insights into the pathogenesis and nonsurgical management of Graves orbitopathy. Nat Rev Endocrinol. 2020;16(2):104‐116. - PubMed

