Efforts toward Synthetic Photosynthesis: Visible Light-Driven CO2 Valorization
- PMID: 37390455
- PMCID: PMC10719901
- DOI: 10.1021/jacs.3c04837
Efforts toward Synthetic Photosynthesis: Visible Light-Driven CO2 Valorization
Abstract
Current methods of urethane preparation from amines invariably involve high-energy and often toxic or cumbersome molecules to make the process exergonic. CO2 aminoalkylation using olefins and amines represents an attractive albeit endergonic alternative. We report a moisture-tolerant method that uses visible light energy to drive this endergonic process (+25 kcal/mol at STP) using sensitized arylcyclohexenes. They convert much of the photon's energy to strain upon olefin isomerization. This strain energy greatly enhances alkene basicity, allowing for sequential protonation by and interception of ammonium carbamates. Following optimization steps and amine scope evaluation, an example product arylcyclohexyl urethane underwent transcarbamoylation with some demonstrative alcohols to form more general urethanes with concomitant regeneration of the arylcyclohexene. This represents a closure of the energetic cycle, producing H2O as the stoichiometric byproduct.
Figures



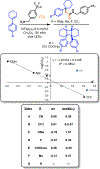
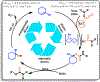
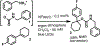
References
-
- Jha SN FACT7359MR https://www.factmr.com/report/polyurethane-market. (accessed 2023-06-14)
-
- Aresta M; Dibenedetto A; Dutta A Energy Issues in the Utilization of CO2 in the Synthesis of Chemicals: The Case of the Direct Carboxylation of Alcohols to Dialkyl-Carbonates. Catal. Today 2017, 281, 345–351. 10.1016/j.cattod.2016.02.046. - DOI
-
- Yoshida M; Hara N; Okuyama S Catalytic Production of Urethanes from Amines and Alkyl Halides in Supercritical Carbon Dioxide. Chem. Commun 2000, 3 (2), 151–152. 10.1039/a908819i. - DOI
-
- Aresta M; Quaranta E Role of the Macrocyclic Polyether in the Synthesis of N-Alkylcarbamate Esters from Primary Amines, CO2 and Alkyl Halides in the Presence of Crown-Ethers. Tetrahedron 1992, 48 (8), 1515–1530. 10.1016/S0040-4020(01)92239-2. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

