Electronic health record data quality assessment and tools: a systematic review
- PMID: 37390812
- PMCID: PMC10531113
- DOI: 10.1093/jamia/ocad120
Electronic health record data quality assessment and tools: a systematic review
Abstract
Objective: We extended a 2013 literature review on electronic health record (EHR) data quality assessment approaches and tools to determine recent improvements or changes in EHR data quality assessment methodologies.
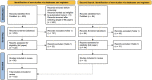
Materials and methods: We completed a systematic review of PubMed articles from 2013 to April 2023 that discussed the quality assessment of EHR data. We screened and reviewed papers for the dimensions and methods defined in the original 2013 manuscript. We categorized papers as data quality outcomes of interest, tools, or opinion pieces. We abstracted and defined additional themes and methods though an iterative review process.
Results: We included 103 papers in the review, of which 73 were data quality outcomes of interest papers, 22 were tools, and 8 were opinion pieces. The most common dimension of data quality assessed was completeness, followed by correctness, concordance, plausibility, and currency. We abstracted conformance and bias as 2 additional dimensions of data quality and structural agreement as an additional methodology.
Discussion: There has been an increase in EHR data quality assessment publications since the original 2013 review. Consistent dimensions of EHR data quality continue to be assessed across applications. Despite consistent patterns of assessment, there still does not exist a standard approach for assessing EHR data quality.
Conclusion: Guidelines are needed for EHR data quality assessment to improve the efficiency, transparency, comparability, and interoperability of data quality assessment. These guidelines must be both scalable and flexible. Automation could be helpful in generalizing this process.
Keywords: clinical research informatics; data quality; electronic health records.
© The Author(s) 2023. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Conflict of interest statement
None declared.
Figures

References
-
- Maddox TM, Rumsfeld JS, Payne PRO.. Questions for artificial intelligence in health care. JAMA 2019; 321 (1): 31–2. - PubMed
-
- Beresniak A, Schmidt A, Proeve J, et al. Cost-benefit assessment of using electronic health records data for clinical research versus current practices: contribution of the Electronic Health Records for Clinical Research (EHR4CR) European Project. Contemp Clin Trials 2016; 46: 85–91. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

