Cortical somatostatin interneuron subtypes form cell-type-specific circuits
- PMID: 37390821
- PMCID: PMC11645782
- DOI: 10.1016/j.neuron.2023.05.032
Cortical somatostatin interneuron subtypes form cell-type-specific circuits
Abstract
The cardinal classes are a useful simplification of cortical interneuron diversity, but such broad subgroupings gloss over the molecular, morphological, and circuit specificity of interneuron subtypes, most notably among the somatostatin interneuron class. Although there is evidence that this diversity is functionally relevant, the circuit implications of this diversity are unknown. To address this knowledge gap, we designed a series of genetic strategies to target the breadth of somatostatin interneuron subtypes and found that each subtype possesses a unique laminar organization and stereotyped axonal projection pattern. Using these strategies, we examined the afferent and efferent connectivity of three subtypes (two Martinotti and one non-Martinotti) and demonstrated that they possess selective connectivity with intratelecephalic or pyramidal tract neurons. Even when two subtypes targeted the same pyramidal cell type, their synaptic targeting proved selective for particular dendritic compartments. We thus provide evidence that subtypes of somatostatin interneurons form cell-type-specific cortical circuits.
Keywords: cortex; interneurons; intratelencephalic; laminar specificity; monosynaptic rabies tracing; optogenetics; pyramidal neurons; pyramidal tract; reciprocal connectivity; somatostatin; spatial transcriptomics; subtypes.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.F. is a founder of Regel Therapeutics, which has no competing interests with the present manuscript. G.F. is an advisor for Neuron and Annual Review of Neuroscience.
Figures
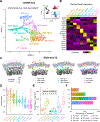




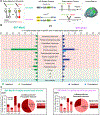
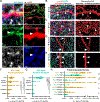

References
-
- Yao Z, van Velthoven CTJ, Nguyen TN, Goldy J, Sedeno-Cortes AE, Baftizadeh F, Bertagnolli D, Casper T, Chiang M, Crichton K, et al. (2021). A taxonomy of transcriptomic cell types across the isocortex and hippocampal formation. Cell 184, 3222–3241.e26. 10.1016/j.cell.2021.04.021. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS074972/NS/NINDS NIH HHS/United States
- R01 NS110079/NS/NINDS NIH HHS/United States
- P30 NS072030/NS/NINDS NIH HHS/United States
- R01 MH071679/MH/NIMH NIH HHS/United States
- R01 NS107257/NS/NINDS NIH HHS/United States
- UG3 MH120096/MH/NIMH NIH HHS/United States
- F31 NS110120/NS/NINDS NIH HHS/United States
- R37 MH071679/MH/NIMH NIH HHS/United States
- R01 NS081297/NS/NINDS NIH HHS/United States
- U19 MH114830/MH/NIMH NIH HHS/United States
- F32 MH125464/MH/NIMH NIH HHS/United States
- R01 NS133751/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

