Therapeutically targeting the consequences of HIV-1-associated gastrointestinal dysbiosis: Implications for neurocognitive and affective alterations
- PMID: 37390973
- PMCID: PMC10494709
- DOI: 10.1016/j.pbb.2023.173592
Therapeutically targeting the consequences of HIV-1-associated gastrointestinal dysbiosis: Implications for neurocognitive and affective alterations
Abstract
Approximately 50 % of the individuals living with human immunodeficiency virus type 1 (HIV-1) are plagued by debilitating neurocognitive impairments (NCI) and/or affective alterations. Sizeable alterations in the composition of the gut microbiome, or gastrointestinal dysbiosis, may underlie, at least in part, the NCI, apathy, and/or depression observed in this population. Herein, two interrelated aims will be critically addressed, including: 1) the evidence for, and functional implications of, gastrointestinal microbiome dysbiosis in HIV-1 seropositive individuals; and 2) the potential for therapeutically targeting the consequences of this dysbiosis for the treatment of HIV-1-associated NCI and affective alterations. First, gastrointestinal microbiome dysbiosis in HIV-1 seropositive individuals is characterized by decreased alpha (α) diversity, a decreased relative abundance of bacterial species belonging to the Bacteroidetes phylum, and geographic-specific alterations in Bacillota (formerly Firmicutes) spp. Fundamentally, changes in the relative abundance of Bacteroidetes and Bacillota spp. may underlie, at least in part, the deficits in γ-aminobutyric acid and serotonin neurotransmission, as well as prominent synaptodendritic dysfunction, observed in this population. Second, there is compelling evidence for the therapeutic utility of targeting synaptodendritic dysfunction as a method to enhance neurocognitive function and improve motivational dysregulation in HIV-1. Further research is needed to determine whether the therapeutics enhancing synaptic efficacy exert their effects by altering the gut microbiome. Taken together, understanding gastrointestinal microbiome dysbiosis resulting from chronic HIV-1 viral protein exposure may afford insight into the mechanisms underlying HIV-1-associated neurocognitive and/or affective alterations; mechanisms which can be subsequently targeted via novel therapeutics.
Keywords: Apathy; Brain-gut-microbiota Axis; Depression; HIV-1-associated neurocognitive disorders; Neurotransmission; Synaptic dysfunction.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no conflict of interest.
Figures
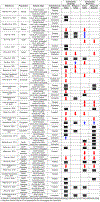
 indicates no statistically significant difference between HIV-1 seropositive individuals and controls, whereas the
indicates no statistically significant difference between HIV-1 seropositive individuals and controls, whereas the  and
and  illustrate significant decreases and increases, respectively, in α diversity in HIV-1 seropositive individuals relative to case-controls.
illustrate significant decreases and increases, respectively, in α diversity in HIV-1 seropositive individuals relative to case-controls.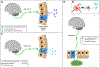

References
-
- Adle-Biassette H, Chrétien F, Wingertsmann L, Héry C, Ereau T, Scaravilli F, Tardieu M, Gray F 1999. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol. 25, 123–133. 10.1046/j.1365-2990.1999.00167.x - DOI - PubMed
-
- Ancona G, Merlini E, Tincati C, Barassi A, Calcagno A, Augello M, Bono V, Bai F, Cannizzo ES, d’Arminio Monforte A, Marchetti G 2021. Long-term suppressive cART is not sufficient to restore intestinal permeability and gut microbiota compositional changes. Front Immunol. 12, 639291. 10.3389/fimmu.2021.639291. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

