Multinuclear 1D and 2D NMR with 19F-Photo-CIDNP hyperpolarization in a microfluidic chip with untuned microcoil
- PMID: 37391397
- PMCID: PMC10313780
- DOI: 10.1038/s41467-023-39537-8
Multinuclear 1D and 2D NMR with 19F-Photo-CIDNP hyperpolarization in a microfluidic chip with untuned microcoil
Abstract
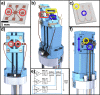
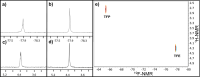
Nuclear Magnetic Resonance (NMR) spectroscopy is a most powerful molecular characterization and quantification technique, yet two major persistent factors limit its more wide-spread applications: poor sensitivity, and intricate complex and expensive hardware required for sophisticated experiments. Here we show NMR with a single planar-spiral microcoil in an untuned circuit with hyperpolarization option and capability to execute complex experiments addressing simultaneously up to three different nuclides. A microfluidic NMR-chip in which the 25 nL detection volume can be efficiently illuminated with laser-diode light enhances the sensitivity by orders of magnitude via photochemically induced dynamic nuclear polarization (photo-CIDNP), allowing rapid detection of samples in the lower picomole range (normalized limit of detection at 600 MHz, nLODf,600, of 0.01 nmol Hz1/2). The chip is equipped with a single planar microcoil operating in an untuned circuit that allows different Larmor frequencies to be addressed simultaneously, permitting advanced hetero-, di- and trinuclear, 1D and 2D NMR experiments. Here we show NMR chips with photo-CIDNP and broadband capabilities addressing two of the major limiting factors of NMR, by enhancing sensitivity as well as reducing cost and hardware complexity; the performance is compared to state-of-the-art instruments.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Giunta, C. J. & Mainz, V. V. Discovery of Nuclear Magnetic Resonance: Rabi, Purcell, and Bloch. in ACS Symposium Series (eds. Strom, E. T. & Mainz, V. V.) vol. 1349 3–20 (American Chemical Society, 2020).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

