Differential intracellular trafficking of extracellular vesicles in microglia and astrocytes
- PMID: 37391572
- PMCID: PMC10313565
- DOI: 10.1007/s00018-023-04841-5
Differential intracellular trafficking of extracellular vesicles in microglia and astrocytes
Abstract
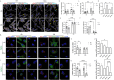
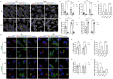
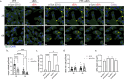
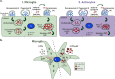
Extracellular vesicles (EVs) have emerged as key players in cell-to-cell communication in both physiological and pathological processes in the Central Nervous System. Thus far, the intracellular pathways involved in uptake and trafficking of EVs within different cell types of the brain are poorly understood. In our study, the endocytic processes and subcellular sorting of EVs were investigated in primary glial cells, particularly linked with the EV-associated α-synuclein (α-syn) transmission. Mouse microglia and astrocytic primary cultures were incubated with DiI-stained mouse brain-derived EVs. The internalization and trafficking pathways were analyzed in cells treated with pharmacological reagents that block the major endocytic pathways. Brain-derived EVs were internalized by both glial cell types; however, uptake was more efficient in microglia than in astrocytes. Colocalization of EVs with early and late endocytic markers (Rab5, Lamp1) indicated that EVs are sorted to endo-lysosomes for subsequent processing. Blocking actin-dependent phagocytosis and/or macropinocytosis with Cytochalasin D or EIPA inhibited EV entry into glial cells, whereas treatment with inhibitors that strip cholesterol off the plasma membrane, induced uptake, however differentially altered endosomal sorting. EV-associated fibrillar α-Syn was efficiently internalized and detected in Rab5- and Lamp1-positive compartments within microglia. Our study strongly suggests that EVs enter glial cells through phagocytosis and/or macropinocytosis and are sorted to endo-lysosomes for subsequent processing. Further, brain-derived EVs serve as scavengers and mediate cell-to-glia transfer of pathological α-Syn which is also targeted to the endolysosomal pathway, suggesting a beneficial role in microglia-mediated clearance of toxic protein aggregates, present in numerous neurodegenerative diseases.
Keywords: Alpha-synuclein; Astrocytes; Endocytosis; Endolysosomal pathway; Lysosome; Microglia; Small extracellular vesicles (sEVs).
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures









References
-
- EL Andaloussi S, Mäger I, Breakefield XO, et al. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 2013; 12: 347–357. doi:10.1038/nrd3978 - PubMed
-
- Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

