Efficacy and Safety of Pirfenidone in Advanced Versus Non-Advanced Idiopathic Pulmonary Fibrosis: Post-Hoc Analysis of Six Clinical Studies
- PMID: 37391667
- PMCID: PMC10427557
- DOI: 10.1007/s12325-023-02565-3
Efficacy and Safety of Pirfenidone in Advanced Versus Non-Advanced Idiopathic Pulmonary Fibrosis: Post-Hoc Analysis of Six Clinical Studies
Abstract
Introduction: In the European Union (EU), the indication for the antifibrotic pirfenidone prior to April 2023 did not include patients with advanced idiopathic pulmonary fibrosis (IPF). This analysis compared the efficacy and safety of pirfenidone in advanced IPF versus non-advanced IPF.
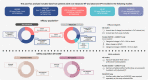
Methods: Data were included from the following studies of pirfenidone: ASCEND (NCT01366209); CAPACITY (004 [NCT00287716] and 006 [NCT00287729]); RECAP (NCT00662038; advanced IPF defined as percent predicted forced vital capacity [%FVC] < 50% and/or percent predicted carbon monoxide diffusing capacity [%DLco] < 35% at baseline); PASSPORT (NCT02699879; advanced IPF defined as baseline %FVC < 50%); and SP-IPF (NCT02951429; patients with advanced IPF [defined as %DLco ≤ 40% at screening] at risk of group 3 pulmonary hypertension).
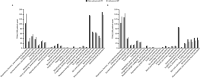
Results: In the pooled ASCEND/CAPACITY studies, the annual mean rate of FVC decline from baseline to Week 52 was significantly lower for pirfenidone versus placebo in advanced (p = 0.0035) and non-advanced IPF (p = 0.0001). Rate of all-cause mortality over 52 weeks was numerically lower for pirfenidone versus placebo in advanced and non-advanced IPF. In RECAP, the mean annual rate of FVC decline from baseline to Week 180 of pirfenidone treatment was similar in patients with advanced (- 141.5 mL) and non-advanced IPF (- 153.5 mL). In SP-IPF, the mean annual rate of FVC decline and rate of all-cause mortality from baseline to Week 52 in patients treated with placebo + pirfenidone were - 93.0 mL and 20.2%, respectively. No new safety signals were identified, and the safety profile of pirfenidone in patients with advanced IPF was generally consistent with that of non-advanced IPF.
Conclusions: These results highlight the benefit of pirfenidone treatment in patients with advanced and non-advanced IPF. As such, the indication for pirfenidone in the EU has now been updated to include the treatment of adult patients with advanced IPF.
Trial registrations: ASCEND (NCT01366209), CAPACITY 004 (NCT00287716), CAPACITY 006 (NCT00287729), RECAP (NCT00662038), PASSPORT (NCT02699879), and SP-IPF (NCT02951429).
Keywords: Advanced idiopathic pulmonary fibrosis; Non-advanced idiopathic pulmonary fibrosis; Pirfenidone.
© 2023. The Author(s).
Conflict of interest statement
Jürgen Behr reports sponsorship or research funds from BMBF, DFG, and LMU-KUM, and has received payment or other financial remuneration from Actelion, AstraZeneca, Bayer, Biogen, Bristol Myers Squibb, Boehringer Ingelheim, F. Hoffmann-La Roche, Ltd., Ferrer, Galapagos, MSD, Novartis, and Promedior, Inc. (acquired by Roche in February 2020). Carlo Albera has served as a consultant for, and received speakers’ bureau fees from, Boehringer Ingelheim, F. Hoffmann-La Roche, and FibroGen. Ulrich Costabel reports personal fees as a consultant or speaker from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, F. Hoffmann-La Roche, Ltd./Genentech, Inc., FibroGen, Galapagos, Novartis, and Pliant Therapeutics. Marilyn K. Glassberg receives research funding from, and has served on advisory boards for, Boehringer Ingelheim, Bristol Myers Squibb, Esperion Therapeutics, F. Hoffmann-La Roche, Ltd./Genentech, Inc., Redx Pharma, and Surrozen, and has served on a steering committee for Bellerophon. Harold Haller Jr has served as a consultant or speaker for Boehringer Ingelheim, Mylan Specialties, and United Therapeutics, and has served as an investigator for Boehringer Ingelheim and United Therapeutics. Steven D. Nathan has served as a consultant for, received speakers’ bureau fees from, and received research funding from Bellerophon, Boehringer Ingelheim, F. Hoffmann-La Roche, Ltd., Galapagos, and United Therapeutics. Wim A. Wuyts reports grants paid to his institution by Boehringer Ingelheim, F. Hoffman-La Roche, Ltd., and Galapagos. Lisa Lancaster has served as a consultant for AstraZeneca, Bellerophon Therapeutics, Daewoong Pharmaceutical, Daiichi Sankyo, DevPro Biopharma, F. Hoffmann-La Roche, Ltd., Nashville Biosciences, Oxygenium, Pieris Pharmaceuticals, Senwha Biosciences, United Therapeutics and Veracyte; has served as a researcher for Boehringer Ingelheim, Bridge Biosciences, Bristol Myers Squibb, Celgene, CSL Behring, Fibrogen, Galecto Biotech, Genentech, Inc., Horizon Therapeutics, NeRRe Therapeutics, Novartis, and Pliant Therapeutics; and has been involved in disease state education with Boehringer Ingelheim, United Therapeutics, and Veractye. Giuseppe Alvaro, Frank Gilberg, and Katerina Samara are employees of F. Hoffmann-La Roche, Ltd.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

