Smart Nanozymes for Cancer Therapy: The Next Frontier in Oncology
- PMID: 37392379
- PMCID: PMC11481082
- DOI: 10.1002/adhm.202300768
Smart Nanozymes for Cancer Therapy: The Next Frontier in Oncology
Abstract
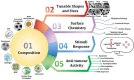
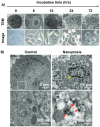
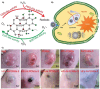
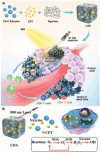
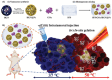
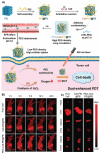
Nanomaterials that mimic the catalytic activity of natural enzymes in the complex biological environment of the human body are called nanozymes. Recently, nanozyme systems have been reported with diagnostic, imaging, and/or therapeutic capabilities. Smart nanozymes strategically exploit the tumor microenvironment (TME) by the in situ generation of reactive species or by the modulation of the TME itself to result in effective cancer therapy. This topical review focuses on such smart nanozymes for cancer diagnosis, and therapy modalities with enhanced therapeutic effects. The dominant factors that guide the rational design and synthesis of nanozymes for cancer therapy include an understanding of the dynamic TME, structure-activity relationships, surface chemistry for imparting selectivity, and site-specific therapy, and stimulus-responsive modulation of nanozyme activity. This article presents a comprehensive analysis of the subject including the diverse catalytic mechanisms of different types of nanozyme systems, an overview of the TME, cancer diagnosis, and synergistic cancer therapies. The strategic application of nanozymes in cancer treatment can well be a game changer in future oncology. Moreover, recent developments may pave the way for the deployment of nanozyme therapy into other complex healthcare challenges, such as genetic diseases, immune disorders, and ageing.
Keywords: cancer; catalytic activities; nanotechnology; nanozymes; oncology; therapies; tumor microenvironments.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

