Single-molecule imaging of genome maintenance proteins encountering specific DNA sequences and structures
- PMID: 37392578
- PMCID: PMC10989508
- DOI: 10.1016/j.dnarep.2023.103528
Single-molecule imaging of genome maintenance proteins encountering specific DNA sequences and structures
Abstract
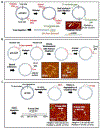
DNA repair pathways are tightly regulated processes that recognize specific hallmarks of DNA damage and coordinate lesion repair through discrete mechanisms, all within the context of a three-dimensional chromatin landscape. Dysregulation or malfunction of any one of the protein constituents in these pathways can contribute to aging and a variety of diseases. While the collective action of these many proteins is what drives DNA repair on the organismal scale, it is the interactions between individual proteins and DNA that facilitate each step of these pathways. In much the same way that ensemble biochemical techniques have characterized the various steps of DNA repair pathways, single-molecule imaging (SMI) approaches zoom in further, characterizing the individual protein-DNA interactions that compose each pathway step. SMI techniques offer the high resolving power needed to characterize the molecular structure and functional dynamics of individual biological interactions on the nanoscale. In this review, we highlight how our lab has used SMI techniques - traditional atomic force microscopy (AFM) imaging in air, high-speed AFM (HS-AFM) in liquids, and the DNA tightrope assay - over the past decade to study protein-nucleic acid interactions involved in DNA repair, mitochondrial DNA replication, and telomere maintenance. We discuss how DNA substrates containing specific DNA sequences or structures that emulate DNA repair intermediates or telomeres were generated and validated. For each highlighted project, we discuss novel findings made possible by the spatial and temporal resolution offered by these SMI techniques and unique DNA substrates.
Keywords: Cohesin; High-speed AFM imaging; Mitochondrial DNA replication; Protein-DNA interactions; Shelterin; Single-molecule imaging.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Friedberg EC (2008) A brief history of the DNA repair field. Cell Res 18, 3–7 - PubMed
-
- Kunkel TA, and Erie DA (2005) DNA mismatch repair. Annu Rev Biochem 74, 681–710 - PubMed
-
- Iyer RR, Pluciennik A, Burdett V, and Modrich PL (2006) DNA mismatch repair: functions and mechanisms. Chem Rev 106, 302–323 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

