Human senescent fibroblasts trigger progressive lung fibrosis in mice
- PMID: 37393107
- PMCID: PMC10415539
- DOI: 10.18632/aging.204825
Human senescent fibroblasts trigger progressive lung fibrosis in mice
Abstract
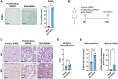
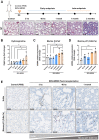
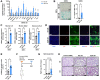
Cell senescence has recently emerged as a potentially relevant pathogenic mechanism in fibrosing interstitial lung diseases (f-ILDs), particularly in idiopathic pulmonary fibrosis. We hypothesized that senescent human fibroblasts may suffice to trigger a progressive fibrogenic reaction in the lung. To address this, senescent human lung fibroblasts, or their secretome (SASP), were instilled into the lungs of immunodeficient mice. We found that: (1) human senescent fibroblasts engraft in the lungs of immunodeficient mice and trigger progressive lung fibrosis associated to increasing levels of mouse senescent cells, whereas non-senescent fibroblasts do not trigger fibrosis; (2) the SASP of human senescent fibroblasts is pro-senescence and pro-fibrotic both in vitro when added to mouse recipient cells and in vivo when delivered into the lungs of mice, whereas the conditioned medium (CM) from non-senescent fibroblasts lacks these activities; and, (3) navitoclax, nintedanib and pirfenidone ameliorate lung fibrosis induced by senescent human fibroblasts in mice, albeit only navitoclax displayed senolytic activity. We conclude that human senescent fibroblasts, through their bioactive secretome, trigger a progressive fibrogenic reaction in the lungs of immunodeficient mice that includes the induction of paracrine senescence in the cells of the host, supporting the concept that senescent cells actively contribute to disease progression in patients with f-ILDs.
Keywords: antifibrotics; cellular senescence; mouse model; pulmonary fibrosis; senolytic.
Conflict of interest statement
Figures


References
-
- Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, Behr J, Cottin V, Danoff SK, Morell F, Flaherty KR, Wells A, Martinez FJ, et al., and American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2018; 198:e44–68. 10.1164/rccm.201807-1255ST - DOI - PubMed

