B-Clear Phase 2b Study Design: Establishing the Efficacy and Safety of Bepirovirsen in Patients with Chronic Hepatitis B Virus Infection
- PMID: 37393402
- PMCID: PMC10427703
- DOI: 10.1007/s12325-023-02531-z
B-Clear Phase 2b Study Design: Establishing the Efficacy and Safety of Bepirovirsen in Patients with Chronic Hepatitis B Virus Infection
Abstract
Introduction: Bepirovirsen (GSK3228836) is an antisense oligonucleotide that induced rapid and prolonged hepatitis B surface antigen (HBsAg) reduction with a favorable safety profile following 4 weeks of treatment in participants with chronic hepatitis B virus (HBV) infection. The objective of the phase 2b study B-Clear is to access the efficacy and safety of bepirovirsen in participants with chronic HBV infection.
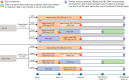
Methods: B-Clear is a phase 2b, multicenter, randomized, partial-blind (sponsor/participant-blinded, investigator-unblinded) study in participants with chronic HBV infection receiving stable nucleos(t)ide analogue (On-NA) or not currently receiving NA therapy (Not-on-NA). Eligibility criteria included HBsAg > 100 IU/mL, HBV DNA < 90 IU/mL (On-NA) or > 2000 IU/mL (Not-on-NA), and alanine aminotransferase ≤ 2 × upper limit of normal (ULN; On-NA) or < 3 × ULN (Not-on-NA). Participants were randomized 3:3:3:1 to one of four treatment arms, with treatment administered weekly as subcutaneous injections with or without loading doses (LD) on days 4 and 11: bepirovirsen 300 mg (with 300 mg LD) for 24 weeks; bepirovirsen 300 mg (with 300 mg LD) for 12 weeks then bepirovirsen 150 mg for 12 weeks; bepirovirsen 300 mg (with 300 mg LD) for 12 weeks then placebo for 12 weeks; placebo for 12 weeks (with placebo LD) then bepirovirsen 300 mg without LD for 12 weeks.
Planned outcomes: The primary endpoint of the study was HBsAg < lower limit of detection and HBV DNA < lower limit of quantification for 24 weeks after the end of bepirovirsen treatment in the absence of rescue medication. The study enrolled 457 participants (On-NA, n = 227; Not-on-NA, n = 230) and the last patient visit occurred in March 2022. The novel design of the B-Clear study will allow assessment of HBsAg and HBV DNA seroclearance post bepirovirsen treatment discontinuation in the presence and absence of background NA therapy.
Trial registration number: ClinicalTrials.gov (NCT04449029; GSK study 209668).
Keywords: Antisense oligonucleotide; Bepirovirsen; Functional cure; GSK3228826; Hepatitis B surface antigen; Nucleos(t)ide analogues; Trial design.
© 2023. The Author(s).
Conflict of interest statement
Jennifer Cremer, Fiona M. Campbell, Robert Elston, Stuart Kendrick, Melanie Paff, Geoff Quinn, and Dickens Theodore are employees of GSK and hold GSK shares.
Figures
References
-
- World Health Organization. Hepatitis B - Key facts 2021 https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. Accessed 6 June 2023.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials