Adrenal gland response to adrenocorticotropic hormone is intact in patients with postural orthostatic tachycardia syndrome
- PMID: 37393658
- PMCID: PMC11409324
- DOI: 10.1016/j.autneu.2023.103105
Adrenal gland response to adrenocorticotropic hormone is intact in patients with postural orthostatic tachycardia syndrome
Abstract
Background: Many patients with postural orthostatic tachycardia syndrome (POTS) are hypovolemic with plasma volume deficits of 10-30 %. Some also have low levels of aldosterone and diminished aldosterone-renin ratios despite elevations in angiotensin II, pointing to potential adrenal dysfunction. To assess adrenal gland responsiveness in POTS, we measured circulating levels of aldosterone and cortisol following adrenocorticotropin hormone (ACTH) stimulation.
Methods: While on a low Na+ diet (∼10 mEq/day), 8 female patients with POTS and 5 female healthy controls (HC) received a low dose (1 μg) ACTH bolus following a baseline blood sample. After 60 min, a high dose (249 μg) infusion of ACTH was administered to ensure maximal adrenal response. Venous aldosterone and cortisol levels were sampled every 30 min for 2 h.
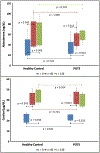
Results: Aldosterone increased in both groups in response to ACTH but was not different between POTS vs. HC at 60 min (53.5 ng/dL [37.8-61.8 ng/dL] vs. 46.1 ng/dL [36.7-84.9 ng/dL]; P = 1.000) or maximally (56.4 ng/dL [49.2-67.1 ng/dL] vs. 49.5 ng/dL [39.1-82.8 ng/dL]; P = 0.524). Cortisol increased in both groups in response to ACTH but was not different in patients with POTS vs. HC at 60 min (39.9 μg/dL [36.1-47.7 μg/dL] vs. 39.3 μg/dL [35.4-46.6 μg/dL]; P = 0.724) or maximally (39.9 μg/dL [33.9-45.4 μg/dL] vs. 42.0 μg/dL [37.6-49.7 μg/dL]; P = 0.354).
Conclusions: ACTH appropriately increased the aldosterone and cortisol levels in patients with POTS. These findings suggest that the response of the adrenal cortex to hormonal stimulation is intact in patients with POTS.
Trial registration: ClinicalTrials.gov NCT01547117.
Keywords: ACTH; Adrenal gland; Aldosterone; Cortisol; Postural orthostatic tachycardia syndrome; RAAS.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest CAS has served as a consultant for Lundbeck NA Ltd. and Theravance RSS. IB has served as a consultant for Lundbeck NA Ltd., Theravance RSS, Ammeal Pharmaceuticals, Regeneron, and Takeda Pharmaceutical. Patent holder of automated binder for treatment of Orthostatic Hypotension. RSS is a Cardiac Arrhythmia Network of Canada (CANet) Network Investigator. SRR is a consultant to Lundbeck LLC, Theravance Biopharma Inc., and Amneal Pharma related to neurogenic orthostatic hypotension; Consultant to Servier Affaires Medicales, Regeneron, argenx BV, and Antag Pharma related to postural orthostatic tachycardia syndrome. Honoraria from Spire Learning and Medscape for developing CME materials on neurogenic orthostatic hypotension. DMSB Chair for a Phase 2 study of an irritable bowel syndrome medication for Arena Pharmaceuticals with compensation. Past-President of the American Autonomic Society without financial compensation. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
References
-
- Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. Jun 2015;12(6):e41–63. doi:10.1016/j.hrthm.2015.03.029 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

