Evolution and Variations of the Ovine Model of Spina Bifida
- PMID: 37393899
- PMCID: PMC10757987
- DOI: 10.1159/000531750
Evolution and Variations of the Ovine Model of Spina Bifida
Abstract
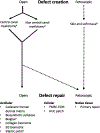
Spina bifida is the most common congenital anomaly of the central nervous system and the first non-fatal fetal lesions to be addressed by fetal intervention. While research in spina bifida has been performed in rodent, nonhuman primate, and canine models, sheep have been a model organism for the disease. This review summarizes the history of development of the ovine model of spina bifida, previous applications, and translation into clinical studies. Initially used by Meuli et al. [Nat Med. 1995;1(4):342-7], fetal myelomeningocele defect creation and in utero repair demonstrated motor function preservation. The addition of myelotomy in this model can reproduce hindbrain herniation malformations, which is the leading cause of mortality and morbidity in humans. Since inception, the ovine models have been validated numerous times as the ideal large animal model for fetal repair, with both locomotive scoring and spina bifida defect scoring adding to the rigor of this model. The ovine model has been used to study different methods of myelomeningocele defect repair, the application of various tissue engineering techniques for neuroprotection and bowel and bladder function. The results of these large animal studies have been translated into human clinical trials including Management of Meningocele Study (MOMS) trial that established current standard of care for prenatal repair of spina bifida defects, and the ongoing trials including the Cellular Therapy for In Utero Repair of Myelomeningocele (CuRe) trial using a stem cell patch for repair. The advancement of these life savings and life-altering therapies began in sheep models, and this notable model continues to be used to further the field including current work with stem cell therapy.
Keywords: Myelomeningocele; Spina bifida; Surgical model.
© 2023 S. Karger AG, Basel.
Conflict of interest statement
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Figures



References
-
- Centers for Disease Control and Prevention (CDC). Racial/ethnic differences in the birth prevalence of spina bifida - United States, 1995–2005. MMWR Morb Mortal Wkly Rep 2009;57:1409–13. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

