Planned delivery or expectant management for late preterm pre-eclampsia in low-income and middle-income countries (CRADLE-4): a multicentre, open-label, randomised controlled trial
- PMID: 37393919
- PMCID: PMC11667733
- DOI: 10.1016/S0140-6736(23)00688-8
Planned delivery or expectant management for late preterm pre-eclampsia in low-income and middle-income countries (CRADLE-4): a multicentre, open-label, randomised controlled trial
Abstract
Background: Pre-eclampsia is a leading cause of maternal and perinatal mortality. Evidence regarding interventions in a low-income or middle-income setting is scarce. We aimed to evaluate whether planned delivery between 34+ 0 and 36+ 6 weeks' gestation can reduce maternal mortality and morbidity without increasing perinatal complications in India and Zambia.
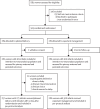
Methods: In this parallel-group, multicentre, open-label, randomised controlled trial, we compared planned delivery versus expectant management in women with pre-eclampsia from 34+ 0 to 36+ 6 weeks' gestation. Participants were recruited from nine hospitals and referral facilities in India and Zambia and randomly assigned to planned delivery or expectant management in a 1:1 ratio by a secure web-based randomisation facility hosted by MedSciNet. Randomisation was stratified by centre and minimised by parity, single-fetus pregnancy or multi-fetal pregnancy, and gestational age. The primary maternal outcome was a composite of maternal mortality or morbidity with a superiority hypothesis. The primary perinatal outcome was a composite of one or more of: stillbirth, neonatal death, or neonatal unit admission of more than 48 h with a non-inferiority hypothesis (margin of 10% difference). Analyses were by intention to treat, with an additional per-protocol analysis for the perinatal outcome. The trial was prospectively registered with ISRCTN, 10672137. The trial is closed to recruitment and all follow-up has been completed.
Findings: Between Dec 19, 2019, and March 31, 2022, 565 women were enrolled. 284 women (282 women and 301 babies analysed) were allocated to planned delivery and 281 women (280 women and 300 babies analysed) were allocated to expectant management. The incidence of the primary maternal outcome was not significantly different in the planned delivery group (154 [55%]) compared with the expectant management group (168 [60%]; adjusted risk ratio [RR] 0·91, 95% CI 0·79 to 1·05). The incidence of the primary perinatal outcome by intention to treat was non-inferior in the planned delivery group (58 [19%]) compared with the expectant management group (67 [22%]; adjusted risk difference -3·39%, 90% CI -8·67 to 1·90; non-inferiority p<0·0001). The results from the per-protocol analysis were similar. There was a significant reduction in severe maternal hypertension (adjusted RR 0·83, 95% CI 0·70 to 0·99) and stillbirth (0·25, 0·07 to 0·87) associated with planned delivery. There were 12 serious adverse events in the planned delivery group and 21 in the expectant management group.
Interpretation: Clinicians can safely offer planned delivery to women with late preterm pre-eclampsia, in a low-income or middle-income country. Planned delivery reduces stillbirth, with no increase in neonatal unit admissions or neonatal morbidity and reduces the risk of severe maternal hypertension. Planned delivery from 34 weeks' gestation should therefore be considered as an intervention to reduce pre-eclampsia associated mortality and morbidity in these settings.
Funding: UK Medical Research Council and Indian Department of Biotechnology.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests JS is a National Institute for Health Research (NIHR) Senior Investigator and is supported by the NIHR Applied Research Collaboration South London at King's College Hospital NHS Foundation Trust. All other authors declare no competing interests.
Comment in
-
Early delivery for pre-eclampsia might save lives in low-income and middle-income settings.Lancet. 2023 Jul 29;402(10399):350-352. doi: 10.1016/S0140-6736(23)00824-3. Epub 2023 Jun 29. Lancet. 2023. PMID: 37393921 No abstract available.
References
-
- WHO Trends in maternal mortality 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. 2019. https://www.who.int/publications/i/item/9789241516488
-
- Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021;398:341–354. - PubMed
-
- Lawn JE, Blencowe H, Waiswa P, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016;387:587–603. - PubMed
-
- Brown MA, Magee LA, Kenny LC, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. - PubMed
-
- Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks' gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374:979–988. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials