Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: a case report
- PMID: 37393920
- PMCID: PMC10552929
- DOI: 10.1016/S0140-6736(23)00775-4
Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: a case report
Abstract
Background: A genetically engineered pig cardiac xenotransplantation was done on Jan 7, 2022, in a non-ambulatory male patient, aged 57 years, with end-stage heart failure, and on veno-arterial extracorporeal membrane oxygenation support, who was ineligible for an allograft. This report details our current understanding of factors important to the xenotransplantation outcome.
Methods: Physiological and biochemical parameters critical for the care of all heart transplant recipients were collected in extensive clinical monitoring in an intensive care unit. To ascertain the cause of xenograft dysfunction, we did extensive immunological and histopathological studies, including electron microscopy and quantification of porcine cytomegalovirus or porcine roseolovirus (PCMV/PRV) in the xenograft, recipient cells, and tissue by DNA PCR and RNA transcription. We performed intravenous immunoglobulin (IVIG) binding to donor cells and single-cell RNA sequencing of peripheral blood mononuclear cells.
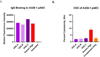
Findings: After successful xenotransplantation, the graft functioned well on echocardiography and sustained cardiovascular and other organ systems functions until postoperative day 47 when diastolic heart failure occurred. At postoperative day 50, the endomyocardial biopsy revealed damaged capillaries with interstitial oedema, red cell extravasation, rare thrombotic microangiopathy, and complement deposition. Increased anti-pig xenoantibodies, mainly IgG, were detected after IVIG administration for hypogammaglobulinaemia and during the first plasma exchange. Endomyocardial biopsy on postoperative day 56 showed fibrotic changes consistent with progressive myocardial stiffness. Microbial cell-free DNA testing indicated increasing titres of PCMV/PRV cell-free DNA. Post-mortem single-cell RNA sequencing showed overlapping causes.
Interpretation: Hyperacute rejection was avoided. We identified potential mediators of the observed endothelial injury. First, widespread endothelial injury indicates antibody-mediated rejection. Second, IVIG bound strongly to donor endothelium, possibly causing immune activation. Finally, reactivation and replication of latent PCMV/PRV in the xenograft possibly initiated a damaging inflammatory response. The findings point to specific measures to improve xenotransplant outcomes in the future.
Funding: The University of Maryland School of Medicine, and the University of Maryland Medical Center.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests DA, AD, TV, LS, and KK are employees of Revivicor, a subsidy of United Therapeutics. KB is an employee of Kiniksa Pharmaceuticals. Members of Program in Cardiac Xenotransplantation received research funding from United Therapeutics. All other authors declare no competing interests.
Figures

















Comment in
-
Possible causes of graft dysfunction in cardiac xenotransplantation.Lancet. 2024 Jun 15;403(10444):2593-2594. doi: 10.1016/S0140-6736(24)00707-4. Lancet. 2024. PMID: 38879254 No abstract available.
-
Possible causes of graft dysfunction in cardiac xenotransplantation - Authors' reply.Lancet. 2024 Jun 15;403(10444):2594-2595. doi: 10.1016/S0140-6736(24)00706-2. Lancet. 2024. PMID: 38879255 No abstract available.
References
-
- Mohiuddin MM, Singh AK, Corcoran PC, Azimzadeh AM, Ayares D, Hoyt RF, et al. B cell depletion by anti CD20 prolongs GalKO.hCD46 pig heterotopic cardiac xenograft survival in baboons. Xenotransplantation. 2009;16(5):357-.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

