Gut barrier-microbiota imbalances in early life lead to higher sensitivity to inflammation in a murine model of C-section delivery
- PMID: 37394428
- PMCID: PMC10316582
- DOI: 10.1186/s40168-023-01584-0
Gut barrier-microbiota imbalances in early life lead to higher sensitivity to inflammation in a murine model of C-section delivery
Erratum in
-
Correction: Gut barrier-microbiota imbalances in early life lead to higher sensitivity to inflammation in a murine model of C-section delivery.Microbiome. 2023 Aug 5;11(1):173. doi: 10.1186/s40168-023-01631-w. Microbiome. 2023. PMID: 37542356 Free PMC article. No abstract available.
Abstract
Background: Most interactions between the host and its microbiota occur at the gut barrier, and primary colonizers are essential in the gut barrier maturation in the early life. The mother-offspring transmission of microorganisms is the most important factor influencing microbial colonization in mammals, and C-section delivery (CSD) is an important disruptive factor of this transfer. Recently, the deregulation of symbiotic host-microbe interactions in early life has been shown to alter the maturation of the immune system, predisposing the host to gut barrier dysfunction and inflammation. The main goal of this study is to decipher the role of the early-life gut microbiota-barrier alterations and its links with later-life risks of intestinal inflammation in a murine model of CSD.
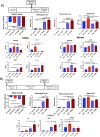
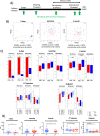
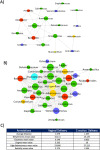
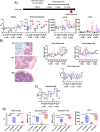
Results: The higher sensitivity to chemically induced inflammation in CSD mice is related to excessive exposure to a too diverse microbiota too early in life. This early microbial stimulus has short-term consequences on the host homeostasis. It switches the pup's immune response to an inflammatory context and alters the epithelium structure and the mucus-producing cells, disrupting gut homeostasis. This presence of a too diverse microbiota in the very early life involves a disproportionate short-chain fatty acids ratio and an excessive antigen exposure across the vulnerable gut barrier in the first days of life, before the gut closure. Besides, as shown by microbiota transfer experiments, the microbiota is causal in the high sensitivity of CSD mice to chemical-induced colitis and in most of the phenotypical parameters found altered in early life. Finally, supplementation with lactobacilli, the main bacterial group impacted by CSD in mice, reverts the higher sensitivity to inflammation in ex-germ-free mice colonized by CSD pups' microbiota.
Conclusions: Early-life gut microbiota-host crosstalk alterations related to CSD could be the linchpin behind the phenotypic effects that lead to increased susceptibility to an induced inflammation later in life in mice. Video Abstract.
Keywords: C-section delivery; Early life; Gut barrier; Inflammation; Microbiota; Murine model; Primary colonization.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Lopetuso LR, Scaldaferri F, Bruno G, Petito V, Franceschi F, Gasbarrini A. The therapeutic management of gut barrier leaking: the emerging role for mucosal barrier protectors. Eur Rev Med Pharmacol Sci. 2015;19:1068–1076. - PubMed
-
- Perrier CCB. Gut permeability and food allergies. Clin Exp Allergy. 2011;41:20–28. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

