SIRT6 is an epigenetic repressor of thoracic aortic aneurysms via inhibiting inflammation and senescence
- PMID: 37394473
- PMCID: PMC10315397
- DOI: 10.1038/s41392-023-01456-x
SIRT6 is an epigenetic repressor of thoracic aortic aneurysms via inhibiting inflammation and senescence
Abstract
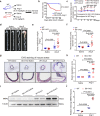
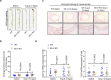
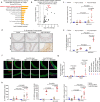
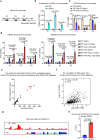
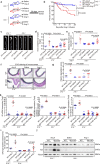
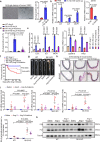
Thoracic aortic aneurysms (TAAs) develop asymptomatically and are characterized by dilatation of the aorta. This is considered a life-threating vascular disease due to the risk of aortic rupture and without effective treatments. The current understanding of the pathogenesis of TAA is still limited, especially for sporadic TAAs without known genetic mutation. Sirtuin 6 (SIRT6) expression was significantly decreased in the tunica media of sporadic human TAA tissues. Genetic knockout of Sirt6 in mouse vascular smooth muscle cells accelerated TAA formation and rupture, reduced survival, and increased vascular inflammation and senescence after angiotensin II infusion. Transcriptome analysis identified interleukin (IL)-1β as a pivotal target of SIRT6, and increased IL-1β levels correlated with vascular inflammation and senescence in human and mouse TAA samples. Chromatin immunoprecipitation revealed that SIRT6 bound to the Il1b promoter to repress expression partly by reducing the H3K9 and H3K56 acetylation. Genetic knockout of Il1b or pharmacological inhibition of IL-1β signaling with the receptor antagonist anakinra rescued Sirt6 deficiency mediated aggravation of vascular inflammation, senescence, TAA formation and survival in mice. The findings reveal that SIRT6 protects against TAA by epigenetically inhibiting vascular inflammation and senescence, providing insight into potential epigenetic strategies for TAA treatment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

