m 6A methylation in cellular senescence of age-associated diseases
- PMID: 37394885
- PMCID: PMC10449638
- DOI: 10.3724/abbs.2023107
m 6A methylation in cellular senescence of age-associated diseases
Abstract
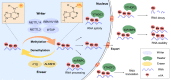
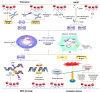
Cellular senescence is a state of irreversible cellular growth arrest that occurs in response to various stresses. In addition to exiting the cell cycle, senescent cells undergo many phenotypic alterations, including metabolic reprogramming, chromatin rearrangement, and senescence-associated secretory phenotype (SASP) development. Furthermore, senescent cells can affect most physiological and pathological processes, such as physiological development; tissue homeostasis; tumour regression; and age-associated disease progression, including diabetes, atherosclerosis, Alzheimer's disease, and hypertension. Although corresponding anti-senescence therapies are actively being explored for the treatment of age-associated diseases, the specific regulatory mechanisms of senescence remain unclear. N 6-methyladenosine (m 6A), a chemical modification commonly distributed in eukaryotic RNA, plays an important role in biological processes such as translation, shearing, and RNA transcription. Numerous studies have shown that m 6A plays an important regulatory role in cellular senescence and aging-related disease. In this review, we systematically summarize the role of m 6A modifications in cellular senescence with regard to oxidative stress, DNA damage, telomere alterations, and SASP development. Additionally, diabetes, atherosclerosis, and Alzheimer's disease regulation via m 6A-mediated cellular senescence is discussed. We further discuss the challenges and prospects of m 6A in cellular senescence and age-associated diseases with the aim of providing rational strategies for the treatment of these age-associated diseases.
Keywords: N -methyladenosine; age-associated diseases; cellular senescence.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Falah G, Giller A, Gutman D, Atzmon G. Breaking the glass ceiling. Gerontology. . 2020;66:309–314. doi: 10.1159/000505995. - DOI - PubMed
-
- Cho SJ, Stout-Delgado HW. Aging and lung disease. Annu Rev Physiol. . 2020;82:433–459. doi: 10.1146/annurev-physiol-021119-034610. - DOI - PMC - PubMed
-
- Rhinn M, Ritschka B, Keyes WM. Cellular senescence in development, regeneration and disease. Development. . 2019;146:dev151837. doi: 10.1242/dev.151837. - DOI - PubMed
-
- Wang WJ, Cai GY, Chen XM. Cellular senescence, senescence-associated secretory phenotype, and chronic kidney disease. Oncotarget. . 2017;8:64520–64533. doi: 10.18632/oncotarget.17327. - DOI - PMC - PubMed
-
- Ferrari S, Pesce M. Stiffness and aging in cardiovascular diseases: the dangerous relationship between force and senescence. Int J Mol Sci. . 2021;22:3404. doi: 10.3390/ijms22073404. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

