Mammary adipocyte flow cytometry as a tool to study mammary gland biology
- PMID: 37394996
- PMCID: PMC10315771
- DOI: 10.1002/2211-5463.13620
Mammary adipocyte flow cytometry as a tool to study mammary gland biology
Abstract
The mammary gland is a vital exocrine organ that has evolved in mammals to secrete milk and provide nutrition to ensure the growth and survival of the neonate The mouse mammary gland displays extraordinary plasticity each time the female undergoes pregnancy and lactation, including a sophisticated process of tertiary branching and alveologenesis to form a branched epithelial tree and subsequently milk-producing alveoli. Upon the cessation of lactation, the gland remodels back to a simple ductal architecture via highly regulated involution processes. At the cellular level, the plasticity is characterised by proliferation of mammary cell populations, differentiation and apoptosis, accompanied by major changes in cell function and morphology. The mammary epithelium requires a specific stromal environment to grow, known as the mammary fat pad. Mammary adipocytes are one of the most prominent cell types in the fat pad, but despite their vast proportion in the tissue and their crucial interaction with epithelial cells, their physiology remains largely unknown. Over the past decade, the need to understand the properties and contribution of mammary adipocytes has become more recognised. However, the development of adequate methods and protocols to study this cellular niche is still lagging, partially due to their fragile nature, the difficulty of isolating them, the lack of reliable cell surface markers and the heterogenous environment in this tissue, which differs from other adipocyte depots. Here, we describe a new rapid and simple flow cytometry protocol specifically designed for the analysis and isolation of mouse mammary adipocytes across mammary gland developmental stages.
Keywords: cell sorting; cell surface staining; flow cytometry; mammary adipocytes; mammary gland.
© 2023 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


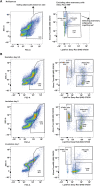
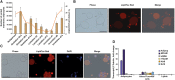
Similar articles
-
Remodeling of Murine Mammary Adipose Tissue during Pregnancy, Lactation, and Involution.J Mammary Gland Biol Neoplasia. 2019 Sep;24(3):207-212. doi: 10.1007/s10911-019-09434-2. Epub 2019 Sep 12. J Mammary Gland Biol Neoplasia. 2019. PMID: 31512027 Free PMC article. Review.
-
Milk protein expression and ductal morphogenesis in the mammary gland in vitro: hormone-dependent and -independent phases of adipocyte-mammary epithelial cell interaction.Dev Biol. 1987 Mar;120(1):245-58. doi: 10.1016/0012-1606(87)90122-9. Dev Biol. 1987. PMID: 3817293
-
The Mammary Gland: Basic Structure and Molecular Signaling during Development.Int J Mol Sci. 2022 Mar 31;23(7):3883. doi: 10.3390/ijms23073883. Int J Mol Sci. 2022. PMID: 35409243 Free PMC article. Review.
-
White, brown and pink adipocytes: the extraordinary plasticity of the adipose organ.Eur J Endocrinol. 2014 Apr 10;170(5):R159-71. doi: 10.1530/EJE-13-0945. Print 2014 May. Eur J Endocrinol. 2014. PMID: 24468979 Review.
-
DIGE based proteome analysis of mammary gland tissue in water buffalo (Bubalus bubalis): lactating vis-a-vis heifer.J Proteomics. 2015 Apr 24;119:100-11. doi: 10.1016/j.jprot.2015.01.018. Epub 2015 Feb 7. J Proteomics. 2015. PMID: 25661041
Cited by
-
FEBS fellowships: supporting excellent science for over four decades.FEBS Open Bio. 2023 Jul;13(7):1138-1139. doi: 10.1002/2211-5463.13659. FEBS Open Bio. 2023. PMID: 37394995 Free PMC article.
References
-
- Watson CJ and Khaled WT (2020) Mammary development in the embryo and adult: new insights into the journey of morphogenesis and commitment. Development 147, dev169862. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

