Single administration vaccines: delivery challenges, in vivo performance, and translational considerations
- PMID: 37395004
- PMCID: PMC10332286
- DOI: 10.1080/14760584.2023.2229431
Single administration vaccines: delivery challenges, in vivo performance, and translational considerations
Abstract
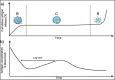
Introduction: With a limited global supply of vaccines and an increasing vaccine hesitancy, improving vaccination coverage has become a priority. Current vaccination regimes require multiple doses to be administered in a defined schedule where missed doses may lead to incomplete vaccine coverage and failure of immunization programmes. As such, there is an ever-increasing demand to convert multi-dose injectable vaccines into single-dose formats, often called single administration vaccines (SAVs).
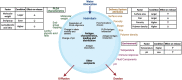
Areas covered: This review summarizes recent developments in the field of SAVs, with a focus on pulsatile or controlled-release formulations. It will identify the technical challenges, translational as well as commercial barriers to SAVs development. Furthermore, the progress of SAV formulations for hepatitis B and polio vaccines will be reviewed thoroughly as case studies, with a focus on the development challenges and the preclinical immunogenicity/reactogenicity data.
Expert opinion: Despite the efforts to develop SAVs, few attempts have advanced to Phase-I trials. Considering the SAV development journey and bottlenecks, including commercial barriers from the early stages, may overcome some of the hurdles around the technology. The renewed global focus on vaccines since the COVID-19 pandemic could facilitate development of a new generation of technologies for pandemic preparedness including strategies for SAVs.
Keywords: Hepatitis B vaccine; PLGA; Single administration vaccines; commercialization; microsphere formulation; multiple dose vaccine; vaccine controlled release; vaccine stability.
Conflict of interest statement
‘Aston University and Avaxzipen have a relevant vaccine formulation technology under development with potential for commercialization. As the corresponding author, I have disclosed those interests fully to Taylor & Francis, and have in place a collaboration agreement for managing any potential conflicts arising from this arrangement. M Prasanna, MK Howard and P Dulal are employees of AVaxziPen. A Walters is currently an employee and shareholder of AstraZeneca. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Figures

References
-
- McHugh KJ, Guarecuco R, Langer R, et al. Single-injection vaccines: progress, challenges, and opportunities. J Control Release. 2015. Dec 10;219:596–609. - PubMed
-
- Riddell NE. Immune responses: primary and secondary. Ency Of Life Sci. 2020; 1(2):316–326. doi: 10.1002/9780470015902.a0029196 - DOI
-
- Immunization coverage by antigen (country, regional, and global trends) [Internet]. UNICEF Data. 2020. [cited 2021 Mar 6]. Available from: https://data.unicef.org/resources/dataset/immunization/#.
-
- Vora LK, Moffatt K, Donnelly RF. 9 - Long-lasting drug delivery systems based on microneedles. In: Larrañeta E, Raghu Raj Singh TDonnelly R, editors. Long-acting drug delivery systems. Woodhead Publishing; 2022. p. 249–287. doi: 10.1016/B978-0-12-821749-8.00010-0 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
