Long-term NAD+ supplementation prevents the progression of age-related hearing loss in mice
- PMID: 37395319
- PMCID: PMC10497810
- DOI: 10.1111/acel.13909
Long-term NAD+ supplementation prevents the progression of age-related hearing loss in mice
Abstract
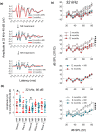
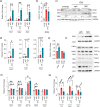
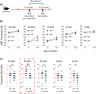
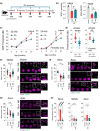
Age-related hearing loss (ARHL) is the most common sensory disability associated with human aging. Yet, there are no approved measures for preventing or treating this debilitating condition. With its slow progression, continuous and safe approaches are critical for ARHL treatment. Nicotinamide Riboside (NR), a NAD+ precursor, is well tolerated even for long-term use and is already shown effective in various disease models including Alzheimer's and Parkinson's disease. It has also been beneficial against noise-induced hearing loss and in hearing loss associated with premature aging. However, its beneficial impact on ARHL is not known. Using two different wild-type mouse strains, we show that long-term NR administration prevents the progression of ARHL. Through transcriptomic and biochemical analysis, we find that NR administration restores age-associated reduction in cochlear NAD+ levels, upregulates biological pathways associated with synaptic transmission and PPAR signaling, and reduces the number of orphan ribbon synapses between afferent auditory neurons and inner hair cells. We also find that NR targets a novel pathway of lipid droplets in the cochlea by inducing the expression of CIDEC and PLIN1 proteins that are downstream of PPAR signaling and are key for lipid droplet growth. Taken together, our results demonstrate the therapeutic potential of NR treatment for ARHL and provide novel insights into its mechanism of action.
Keywords: NAD+; age-related hearing loss; nicotinamide riboside.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
Dr. Vilhelm Bohr previously had a CRADA agreement with ChromaDex.
Figures


References
-
- Briggs, S. E. (2019). Special populations in implantable auditory devices. Geriatric Otolaryngologic Clinics of North America, 52, 331–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

