Effect of Extracellular Ribonucleic Acids on Neurovascularization in Osteoarthritis
- PMID: 37395388
- PMCID: PMC10502862
- DOI: 10.1002/advs.202301763
Effect of Extracellular Ribonucleic Acids on Neurovascularization in Osteoarthritis
Abstract
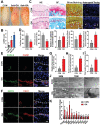
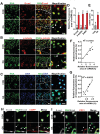
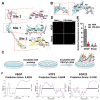
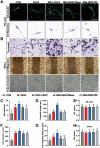
Osteoarthritis is a degenerative disease characterized by abnormal neurovascularization at the osteochondral junctions, the regulatory mechanisms of which remain poorly understood. In the present study, a murine osteoarthritic model with augmented neurovascularization at the osteochondral junction is used to examine this under-evaluated facet of degenerative joint dysfunction. Increased extracellular RNA (exRNA) content is identified in neurovascularized osteoarthritic joints. It is found that the amount of exRNA is positively correlated with the extent of neurovascularization and the expression of vascular endothelial growth factor (VEGF). In vitro binding assay and molecular docking demonstrate that synthetic RNAs bind to VEGF via electrostatic interactions. The RNA-VEGF complex promotes the migration and function of endothelial progenitor cells and trigeminal ganglion cells. The use of VEGF and VEGFR2 inhibitors significantly inhibits the amplification of the RNA-VEGF complex. Disruption of the RNA-VEGF complex by RNase and polyethyleneimine reduces its in vitro activities, as well as prevents excessive neurovascularization and osteochondral deterioration in vivo. The results of the present study suggest that exRNAs may be potential targets for regulating nerve and blood vessel ingrowth under physiological and pathological joint conditions.
Keywords: extracellular RNA; neurovascularization; osteoarthritis; osteochondral junction; vascular endothelial growth factor.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
