PEPseq quantifies transcriptome-wide changes in protein occupancy and reveals selective translational repression after translational stress
- PMID: 37395449
- PMCID: PMC10415142
- DOI: 10.1093/nar/gkad557
PEPseq quantifies transcriptome-wide changes in protein occupancy and reveals selective translational repression after translational stress
Abstract
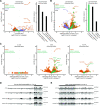
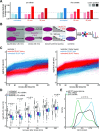
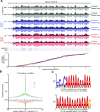
Post-transcriptional gene regulation is accomplished by the interplay of the transcriptome with RNA-binding proteins, which occurs in a dynamic manner in response to altered cellular conditions. Recording the combined occupancy of all proteins binding to the transcriptome offers the opportunity to interrogate if a particular treatment leads to any interaction changes, pointing to sites in RNA that undergo post-transcriptional regulation. Here, we establish a method to monitor protein occupancy in a transcriptome-wide fashion by RNA sequencing. To this end, peptide-enhanced pull-down for RNA sequencing (or PEPseq) uses metabolic RNA labelling with 4-thiouridine (4SU) for light-induced protein-RNA crosslinking, and N-hydroxysuccinimide (NHS) chemistry to isolate protein-crosslinked RNA fragments across all long RNA biotypes. We use PEPseq to investigate changes in protein occupancy during the onset of arsenite-induced translational stress in human cells and reveal an increase of protein interactions in the coding region of a distinct set of mRNAs, including mRNAs coding for the majority of cytosolic ribosomal proteins. We use quantitative proteomics to demonstrate that translation of these mRNAs remains repressed during the initial hours of recovery after arsenite stress. Thus, we present PEPseq as a discovery platform for the unbiased investigation of post-transcriptional regulation.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

