The Emerging Roles of Ferroptosis in Neonatal Diseases
- PMID: 37396013
- PMCID: PMC10312340
- DOI: 10.2147/JIR.S414316
The Emerging Roles of Ferroptosis in Neonatal Diseases
Abstract
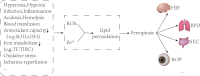
Ferroptosis is a novel type of programmed cell death involved in many diseases' pathological processes. Ferroptosis is characterized by lipid peroxidation, reactive oxygen species accumulation, and iron metabolism disorder. Newborns are susceptible to ferroptosis due to their special physiological state, which is prone to abnormal iron metabolism and the accumulation of reactive oxygen species. Recent studies have linked ferroptosis to a variety of diseases in the neonatal period (including hypoxic-ischemic encephalopathy, bronchopulmonary dysplasia, and necrotizing enterocolitis). Ferroptosis may become an effective target for the treatment of neonatal-related diseases. In this review, the ferroptosis molecular mechanism, metabolism characteristics of iron and reactive oxygen species in infants, the relationship between ferroptosis and common infant disorders, and the treatment of infant diseases targeted for ferroptosis are systematically summarized.
Keywords: ferroptosis; iron metabolism; lipid peroxidation; neonatal disease; reactive oxygen species.
© 2023 Chen et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures

Similar articles
-
From Iron Metabolism to Ferroptosis: Pathologic Changes in Coronary Heart Disease.Oxid Med Cell Longev. 2022 Aug 10;2022:6291889. doi: 10.1155/2022/6291889. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35993022 Free PMC article. Review.
-
Molecular Mechanisms of Iron Mediated Programmed Cell Death and Its Roles in Eye Diseases.Front Nutr. 2022 Apr 5;9:844757. doi: 10.3389/fnut.2022.844757. eCollection 2022. Front Nutr. 2022. PMID: 35495915 Free PMC article. Review.
-
The Potential Role of Ferroptosis in Neonatal Brain Injury.Front Neurosci. 2019 Feb 14;13:115. doi: 10.3389/fnins.2019.00115. eCollection 2019. Front Neurosci. 2019. PMID: 30837832 Free PMC article. Review.
-
Molecular mechanisms of ferroptosis and its roles in leukemia.Front Oncol. 2023 Dec 6;13:1308869. doi: 10.3389/fonc.2023.1308869. eCollection 2023. Front Oncol. 2023. PMID: 38125948 Free PMC article. Review.
-
Ferroptosis: mechanisms and advances in ocular diseases.Mol Cell Biochem. 2023 Sep;478(9):2081-2095. doi: 10.1007/s11010-022-04644-5. Epub 2023 Jan 8. Mol Cell Biochem. 2023. PMID: 36617346 Review.
Cited by
-
Aerosol inhalation of dimeric artesunate phospholipid-conjugated liposomes ameliorates inflammation, fibrosis, and ferroptosis in neonatal mice with hyperoxia-induced lung injury.Front Pharmacol. 2025 Jul 21;16:1542743. doi: 10.3389/fphar.2025.1542743. eCollection 2025. Front Pharmacol. 2025. PMID: 40761396 Free PMC article.
-
Structural and Functional Effects of C5aR1 Antagonism in a Rat Model of Neonatal Hypoxic-Ischemic Encephalopathy.Dev Neurosci. 2025;47(2):112-126. doi: 10.1159/000539506. Epub 2024 May 25. Dev Neurosci. 2025. PMID: 38797164 Free PMC article.
-
Screening for biomarkers of bronchopulmonary dysplasia: a bioinformatics analysis.Transl Pediatr. 2025 Apr 30;14(4):658-670. doi: 10.21037/tp-2024-595. Epub 2025 Apr 27. Transl Pediatr. 2025. PMID: 40386354 Free PMC article.
-
N-Acetylcysteine Alleviates Necrotizing Enterocolitis by Depressing SESN2 Expression to Inhibit Ferroptosis in Intestinal Epithelial Cells.Inflammation. 2025 Feb;48(1):464-482. doi: 10.1007/s10753-024-02068-5. Epub 2024 Jul 22. Inflammation. 2025. PMID: 39037665 Free PMC article.
-
Untargeted lipidomics of bronchopulmonary dysplasia induced by hyperoxia exposure in rats.Transl Pediatr. 2024 May 31;13(5):748-759. doi: 10.21037/tp-23-546. Epub 2024 May 24. Transl Pediatr. 2024. PMID: 38840687 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

