Advancing posaconazole quantification analysis with a new reverse-phase HPLC method in its bulk and marketed dosage form
- PMID: 37396051
- PMCID: PMC10314186
- DOI: 10.12688/f1000research.132841.2
Advancing posaconazole quantification analysis with a new reverse-phase HPLC method in its bulk and marketed dosage form
Abstract
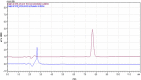
Introduction: Posaconazole is a widely used antifungal drug, and its accurate quantification is essential for quality control and assessment of its pharmaceutical products. This study aimed to develop and validate a reverse-phase high-performance liquid chromatography (HPLC) analytical method for quantifying Posaconazole in bulk and dosage form. Methods: The HPLC method was developed and validated based on International Conference on Harmonisation (ICH) guidelines. The developed method was then applied to quantify Posaconazole in a marketed tablet formulation. The method's specificity, linearity, precision, accuracy, robustness, and stability were evaluated. Results: The developed HPLC method showed good linearity over a 2-20 μg/mL concentration range. The percentage recovery of Posaconazole from the bulk and marketed formulations was found to be 99.01% and 99.05%, respectively. The intra-day and inter-day precisions were less than 1%, and the method was stable under different conditions. The HPLC method was successfully applied to quantify Posaconazole in the marketed formulation. Conclusion: The developed and validated HPLC method is reliable and efficient for analyzing Posaconazole in bulk and dosage forms. The method's accuracy, precision, specificity, linearity, robustness, and stability demonstrate its effectiveness. The method can be used for the quality control and assessment of Posaconazole-containing pharmaceutical products.
Keywords: Analytical method; HPLC; Posaconazole; Quality control; Validation.
Copyright: © 2023 Rama A et al.
Conflict of interest statement
No competing interests were disclosed.
Figures
Similar articles
-
AQbD-guided development and validation of an innovative extraction procedure and stability-indicating RP-HPLC method for quantification of posaconazole in tablet formulation.Ann Pharm Fr. 2024 Nov;82(6):1088-1102. doi: 10.1016/j.pharma.2024.07.003. Epub 2024 Aug 26. Ann Pharm Fr. 2024. PMID: 39002855
-
Application of HPTLC, spectrofluorimetry and differential pulse voltammetry for determination of the antifungal drug posaconazole in suspension dosage form.Ann Pharm Fr. 2019 Sep;77(5):382-393. doi: 10.1016/j.pharma.2019.04.004. Epub 2019 May 25. Ann Pharm Fr. 2019. PMID: 31138437
-
An Eco-Friendly RP-HPLC Method Development and Validation for Quantification of Favipiravir in Bulk and Tablet Dosage Form Followed by Forced Degradation Study.J Chromatogr Sci. 2024 May 31;62(5):432-438. doi: 10.1093/chromsci/bmad093. J Chromatogr Sci. 2024. PMID: 38266038
-
Review of the new delayed-release oral tablet and intravenous dosage forms of posaconazole.Pharmacotherapy. 2015 Feb;35(2):208-19. doi: 10.1002/phar.1533. Epub 2015 Feb 3. Pharmacotherapy. 2015. PMID: 25644669 Review.
-
Overview of Analytical Methods for Evaluating Tinidazole.J AOAC Int. 2023 Mar 1;106(2):309-315. doi: 10.1093/jaoacint/qsac142. J AOAC Int. 2023. PMID: 36355444 Review.
References
-
- Abou El-Alamin MM, Sultan MA, Atia MA, et al. : Novel Application of Pentabromobenzyl Column for Simultaneous Determination of Eight Antifungal Drugs Using High-performance Liquid Chromatography. Comb. Chem. High Throughput Screen. 2020;23(10):991–1001. 10.2174/1386207323666200220114818 - DOI - PubMed
-
- Araújo JIR, Moura FL, Soares MFLR, et al. : Stability study and oxidative degradation kinetics of posaconazole. Microchem. J. 2019;151:104181. 10.1016/j.microc.2019.104181 - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources