Maternal glucose intolerance during pregnancy affects offspring POMC expression and results in adult metabolic alterations in a sex-dependent manner
- PMID: 37396180
- PMCID: PMC10311085
- DOI: 10.3389/fendo.2023.1189207
Maternal glucose intolerance during pregnancy affects offspring POMC expression and results in adult metabolic alterations in a sex-dependent manner
Abstract
Introduction: Gestational diabetes (GDM) is associated with negative outcomes in mothers and their offspring, including greater risks of macrosomia at birth and the development of metabolic disorders. While these outcomes are well-established, the mechanisms by which this increased metabolic vulnerability is conferred on the offspring are comparatively lacking. One proposed mechanism is that maternal glycemic dysregulation alters the development of the hypothalamic regions related to metabolism and energy balance.
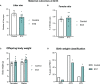
Methods: To investigate this possibility, in this study, we first examined the effects of STZ-induced maternal glucose intolerance on the offspring on pregnancy day (PD) 19, and, in a second experiment, in early adulthood (postnatal day (PND) 60). Whether effects would be influenced by sex, or exposure of offspring to a high-fat diet was also investigated. The impact of maternal STZ treatment on POMC neuron number in the ARC of offspring at both time points was also examined.
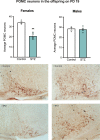
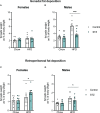
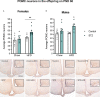
Results: As expected, STZ administration on PD 7 decreased maternal glucose tolerance, and increased risk for macrosomia, and loss of pups at birth. Offspring of STZ-treated mothers were also more vulnerable to developing metabolic impairments in adulthood. These were accompanied by sex-specific effects of maternal STZ treatment in the offspring, including fewer POMC neurons in the ARC of female but not male infants in late pregnancy and a higher number of POMC neurons in the ARC of both male and female adult offspring of STZ-treated dams, which was exacerbated in females exposed to a high-fat diet after weaning.
Discussion: This work suggests that maternal hyperglycemia induced by STZ treatment, in combination with early-life exposure to an obesogenic diet, leads to adult metabolic alterations that correlate with the increased hypothalamic expression of POMC, showing that maternal glycemic dysregulation can impact the development of hypothalamic circuits regulating energy state with a stronger impact on female offspring.
Keywords: POMC; gestational diabetes; glucose tolerance; hypothalamus; metabolic programming; pregnancy; streptozotocin.
Copyright © 2023 Martins, Silver, Ayoub, Hyland, Woodside, Kiss and Abizaid.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Amylin receptor insensitivity impairs hypothalamic POMC neuron differentiation in the male offspring of maternal high-fat diet-fed mice.Mol Metab. 2021 Feb;44:101135. doi: 10.1016/j.molmet.2020.101135. Epub 2020 Dec 3. Mol Metab. 2021. PMID: 33279727 Free PMC article.
-
Effects of maternal mild hyperglycemia associated with snack intake on offspring metabolism and behavior across the lifespan.Physiol Behav. 2024 Mar 15;276:114483. doi: 10.1016/j.physbeh.2024.114483. Epub 2024 Feb 6. Physiol Behav. 2024. PMID: 38331375
-
Epigenomic and metabolic responses of hypothalamic POMC neurons to gestational nicotine exposure in adult offspring.Genome Med. 2016 Sep 8;8(1):93. doi: 10.1186/s13073-016-0348-2. Genome Med. 2016. PMID: 27609221 Free PMC article.
-
Fetal alcohol programming of hypothalamic proopiomelanocortin system by epigenetic mechanisms and later life vulnerability to stress.Alcohol Clin Exp Res. 2014 Sep;38(9):2323-30. doi: 10.1111/acer.12497. Epub 2014 Jul 28. Alcohol Clin Exp Res. 2014. PMID: 25069392 Free PMC article. Review.
-
Screening and diagnosing gestational diabetes mellitus.Evid Rep Technol Assess (Full Rep). 2012 Oct;(210):1-327. Evid Rep Technol Assess (Full Rep). 2012. PMID: 24423035 Free PMC article. Review.
Cited by
-
Maternal Nutritional Environment and the Development of the Melanocortin System.Compr Physiol. 2025 Jun;15(3):e70020. doi: 10.1002/cph4.70020. Compr Physiol. 2025. PMID: 40474775 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

