Electronic Immunoassay Using Enzymatic Metallization on Microparticles
- PMID: 37396256
- PMCID: PMC10308597
- DOI: 10.1021/acsomega.3c01939
Electronic Immunoassay Using Enzymatic Metallization on Microparticles
Abstract
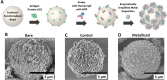
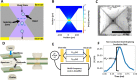
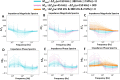
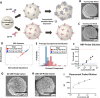
We present here an inexpensive method for generating a sensitive direct electronic readout in bead-based immunoassays without the use of any intermediate optical instrumentation (e.g., lasers, photomultipliers, etc.). Analyte binding to capture antigen-coated beads or microparticles is converted to probe-directed enzymatically amplified silver metallization on microparticle surfaces. Individual microparticles are then rapidly characterized in a high-throughput manner via single-bead multifrequency electrical impedance spectra captured using a simple and inexpensive microfluidic impedance spectrometry system we develop here, where they flow through a three-dimensional (3D)-printed plastic microaperture sandwiched between plated through-hole electrodes on a printed circuit board. Metallized microparticles are found to have unique impedance signatures distinguishing them from unmetallized ones. Coupled with a machine learning algorithm, this enables a simple electronic readout of the silver metallization density on microparticle surfaces and hence the underlying analyte binding. Here, we also demonstrate the use of this scheme to measure the antibody response to the viral nucleocapsid protein in convalescent COVID-19 patient serum.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Huergo L. F.; Selim K. A.; Conzentino M. S.; Gerhardt E. C. M.; Santos A. R. S.; Wagner B.; Alford J. T.; Deobald N.; Pedrosa F. O.; de Souza E. M.; Nogueira M. B.; Raboni S. M.; Souto D.; Rego F. G. M.; Zanette D. L.; Aoki M. N.; Nardin J. M.; Fornazari B.; Morales H. M. P.; Borges V. A.; Nelde A.; Walz J. S.; Becker M.; Schneiderhan-Marra N.; Rothbauer U.; Reis R. A.; Forchhammer K. Magnetic Bead-Based Immunoassay Allows Rapid, Inexpensive, and Quantitative Detection of Human SARS-CoV-2 Antibodies. ACS Sens. 2021, 6, 703–708. 10.1021/acssensors.0c02544. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

