The Realm of Unconventional Noncovalent Interactions in Proteins: Their Significance in Structure and Function
- PMID: 37396257
- PMCID: PMC10308531
- DOI: 10.1021/acsomega.3c00205
The Realm of Unconventional Noncovalent Interactions in Proteins: Their Significance in Structure and Function
Abstract
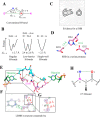
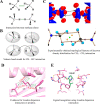
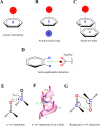
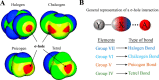
Proteins and their assemblies are fundamental for living cells to function. Their complex three-dimensional architecture and its stability are attributed to the combined effect of various noncovalent interactions. It is critical to scrutinize these noncovalent interactions to understand their role in the energy landscape in folding, catalysis, and molecular recognition. This Review presents a comprehensive summary of unconventional noncovalent interactions, beyond conventional hydrogen bonds and hydrophobic interactions, which have gained prominence over the past decade. The noncovalent interactions discussed include low-barrier hydrogen bonds, C5 hydrogen bonds, C-H···π interactions, sulfur-mediated hydrogen bonds, n → π* interactions, London dispersion interactions, halogen bonds, chalcogen bonds, and tetrel bonds. This Review focuses on their chemical nature, interaction strength, and geometrical parameters obtained from X-ray crystallography, spectroscopy, bioinformatics, and computational chemistry. Also highlighted are their occurrence in proteins or their complexes and recent advances made toward understanding their role in biomolecular structure and function. Probing the chemical diversity of these interactions, we determined that the variable frequency of occurrence in proteins and the ability to synergize with one another are important not only for ab initio structure prediction but also to design proteins with new functionalities. A better understanding of these interactions will promote their utilization in designing and engineering ligands with potential therapeutic value.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Attochemistry of Ionized Halogen, Chalcogen, Pnicogen, and Tetrel Noncovalent Bonded Clusters.J Phys Chem A. 2016 Dec 29;120(51):10057-10071. doi: 10.1021/acs.jpca.6b09813. Epub 2016 Dec 2. J Phys Chem A. 2016. PMID: 28030953
-
Noncovalent Interactions in Organometallic Chemistry: From Cohesion to Reactivity, a New Chapter.Acc Chem Res. 2021 Oct 19;54(20):3828-3840. doi: 10.1021/acs.accounts.1c00393. Epub 2021 Oct 7. Acc Chem Res. 2021. PMID: 34617728
-
Noncovalent Carbon-Bonding Interactions in Proteins.Angew Chem Int Ed Engl. 2018 Dec 10;57(50):16496-16500. doi: 10.1002/anie.201811171. Epub 2018 Nov 12. Angew Chem Int Ed Engl. 2018. PMID: 30347500
-
Non-classical Non-covalent σ-Hole Interactions in Protein Structure and Function: Concepts for Potential Protein Engineering Applications.Chem Asian J. 2023 Apr 3;18(7):e202300026. doi: 10.1002/asia.202300026. Epub 2023 Feb 24. Chem Asian J. 2023. PMID: 36764929 Review.
-
Principles of Cation-π Interactions for Engineering Mussel-Inspired Functional Materials.Acc Chem Res. 2022 Apr 19;55(8):1171-1182. doi: 10.1021/acs.accounts.2c00068. Epub 2022 Mar 28. Acc Chem Res. 2022. PMID: 35344662 Review.
Cited by
-
A novel imatinib analogue inhibitor of chronic myeloid leukaemia: design, synthesis and characterization-explanation of its folded conformation.R Soc Open Sci. 2025 Jan 29;12(1):241654. doi: 10.1098/rsos.241654. eCollection 2025 Jan. R Soc Open Sci. 2025. PMID: 39881787 Free PMC article.
-
Theoretical monitoring of aromaticity induction from noble gases to Borole structure.Sci Rep. 2025 Feb 27;15(1):7043. doi: 10.1038/s41598-025-91459-1. Sci Rep. 2025. PMID: 40016548 Free PMC article.
-
Structural insights into SOD1: from in silico and molecular dynamics to experimental analyses of ALS-associated E49K and R115G mutants.Front Mol Biosci. 2025 Feb 25;12:1532375. doi: 10.3389/fmolb.2025.1532375. eCollection 2025. Front Mol Biosci. 2025. PMID: 40070688 Free PMC article.
-
Larvicidal potential of Knoevenagel adducts against Aedes aegypti: theoretical study and in vitro validation.Future Med Chem. 2025 May;17(9):999-1011. doi: 10.1080/17568919.2025.2498877. Epub 2025 May 5. Future Med Chem. 2025. PMID: 40323274
-
Probing the microRNA landscape in cadmium chloride induced renal toxicity through an in silico approach.Sci Rep. 2025 Jul 12;15(1):25251. doi: 10.1038/s41598-025-11473-1. Sci Rep. 2025. PMID: 40652097 Free PMC article.
References
-
- van der Waals D. J.On the Continuity of the Gaseous and Liquid States; Leiden University: Leiden, The Netherlands, 1873.
-
- Kollman P. A. Noncovalent Interactions. Acc. Chem. Res. 1977, 10 (10), 365–371. 10.1021/ar50118a003. - DOI
-
- Kollman P. Chapter 2 Non-Covalent Forces of Importance in Biochemistry. New Compr.Biochem. 1984, 6, 55–71. 10.1016/S0167-7306(08)60373-7. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

